以异噁唑啉 N-氧化物为中间体的醛醇产品乌姆波隆法
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种非对映选择性合成醛醇的两步umpolung 方法,其中共轭硝基烯用作烯醇阳离子的合成等效物,而硫酰基则用作α-甲醇阴离子的等效物。生成的异噁唑啉 N-氧化物在室温和 1 atm 氢压的温和条件下催化还原裂解成醛醇。通过合成一系列难以用经典醛醇反应合成的多取代 β-羟基酮,证明了该方法的高效性。所开发的方法通过选择具有适当 C,C 双键位置的硝基烯异构体,实现了醛醇的变构组装。本文章由计算机程序翻译,如有差异,请以英文原文为准。
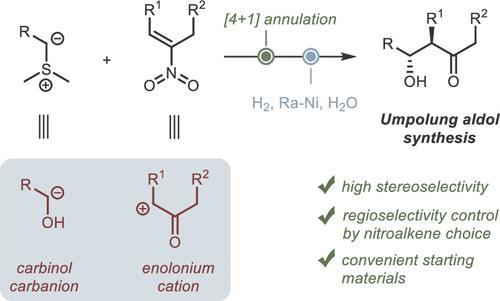
Umpolung Approach to Aldol Products via Isoxazoline N-Oxides as Intermediates
A two-step umpolung approach to the diastereoselective synthesis of aldols was developed, in which a conjugated nitroalkene is used as the synthetic equivalent of the enolonium cation, while a sulfur ylide acts as the equivalent of the α-carbinol anion. The resulting isoxazoline N-oxides undergo catalytic reductive cleavage to aldols under mild conditions at room temperature and under 1 atm hydrogen pressure. The efficiency of the method was demonstrated by the synthesis of a series of polysubstituted β-hydroxyketones that are difficult to synthesize using the classical aldol reaction. The developed approach allows for the regiodivergent assembly of aldols by selecting a nitroalkene isomer with the appropriate position of the C,C double bond.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


