冻干精神嗜性梭菌菌丝体对萜烯的化学酶促环氧化作用 01
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
萜烯环氧化物因其生物活性或在聚合物生产中的用途而成为一类特别重要的化合物。开发一种通用、廉价和可持续的方法,从容易获得的萜烯中获得这些化合物非常重要。用 Cladosporium cladosporioides01 的冻干菌丝体进行化学酶促环氧化是一种替代方法,而不是基于众所周知的、昂贵的来自南极念珠菌的脂肪酶 B 的工艺。在本研究中,我们研究了真菌生物催化剂环氧化柠檬烯的动力学,发现在 "绿色 "溶剂乙酸乙酯中,H2O2 相对于底物过量 4 倍时,环氧化活性最高。环氧化作用遵循一种双向顺序机制,其中主要的初始产物柠檬烯 1,2-环氧化物和次要的中间产物柠檬烯 8,9-环氧化物进一步转化为柠檬烯二环氧化物。底物特异性研究表明,该生物催化剂可将多种线性和环状单萜烯,如芳樟醇、香茅烯、香茅醛、α-蒎烯、β-蒎烯、香茅醇和紫苏醇高效地转化为各自的环氧化物。这种生物催化剂可以在不使用过酸的情况下直接氧化双键。冷冻干燥的菌丝体在脱脂后表现出较高的生物催化活性,在乙酸乙酯中和氧化剂存在时具有较高的稳定性。经过八个生物催化循环后,其活性没有下降。因此,该菌丝体可成功用于萜烯环氧化物的可持续大规模生产。本文章由计算机程序翻译,如有差异,请以英文原文为准。
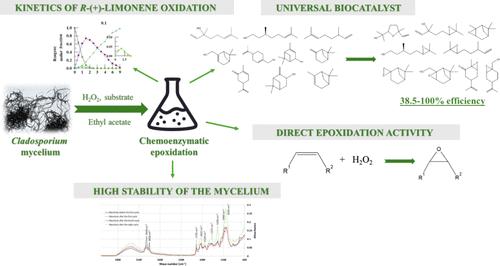
Chemoenzymatic Epoxidation of Terpenes by Lyophilized Mycelium of Psychrophilic Cladosporium cladosporioides 01
Terpene epoxides constitute a group of compounds of particular importance due to their biological activity or use in the production of polymers. It is important to develop a universal, inexpensive, and sustainable method to obtain these compounds from readily available terpenes. Chemoenzymatic epoxidation by the freeze-dried mycelium of Cladosporium cladosporioides01 is an alternative to processes based on the well-known and expensive lipase B fromCandida antarctica. In the present work, we studied the kinetics of limonene epoxidation by the fungal biocatalyst and found the highest epoxidation activity in the “green” solvent ethyl acetate with a 4-fold excess of H2O2 relative to the substrate. Epoxidation followed a two-way sequential mechanism in which the major initial product limonene 1,2-epoxide and the minor intermediate product limonene 8,9-epoxide were further converted to limonene diepoxide. Substrate specificity studies revealed that a wide number of linear and cyclic monoterpenes, e.g., linalool, citronellene, citronellal, α-pinene, β-pinene, myrtenol, and perillyl alcohol, were efficiently converted to their respective epoxides by this biocatalyst. The biocatalyst may show activity in the direct oxidation of a double bond without the use of a peracid. The freeze-dried mycelium exhibited higher biocatalytic activity after defatting and high stability in ethyl acetate and in the presence of an oxidant. No decrease in its activity was observed after eight biocatalytic cycles. Therefore, the mycelium can be successfully used for the sustainable large-scale production of terpene epoxides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


