细胞外植物枯草酶抑制冷冲击肽诱导剂水平
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
识别细胞表面的病原体相关分子模式对植物免疫至关重要。许多这些模式的蛋白质性质表明,分泌蛋白酶在其形成和稳定过程中发挥着重要作用。在这里,我们证明了细胞外亚基酶 SBT5.2a 通过裂解免疫原性 csp22 表位,使细菌性植物病原体 丁香假单胞菌(Pseudomonas syringae)的冷休克蛋白(CSPs)的免疫原性失活。因此,缺乏 SBT5.2a 活性的突变体植物保留了较高水平的 csp22,从而增强了免疫反应并降低了病原体的生长。SBT5.2 的敏感性受裂解位点周围序列变异的影响,并可能延伸到其他细菌物种的 CSP。这些发现表明,细菌病原体之间 csp22 稳定性的变化是植物与细菌相互作用的关键因素,病原体利用植物蛋白酶来避免模式识别。本文章由计算机程序翻译,如有差异,请以英文原文为准。
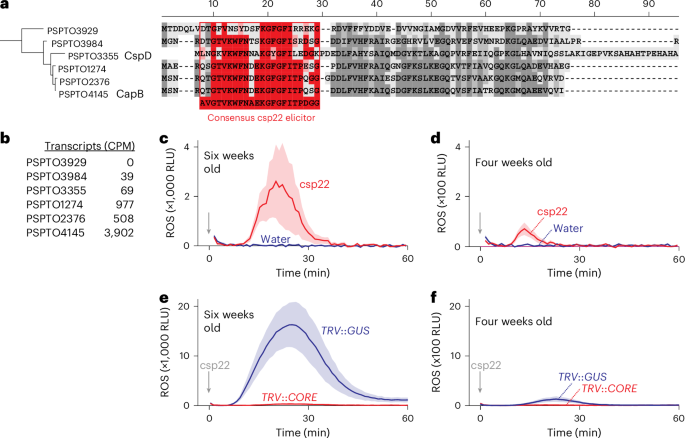
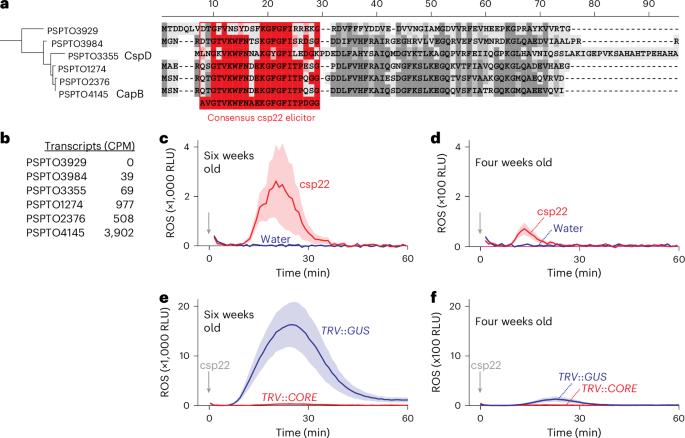
Extracellular plant subtilases dampen cold-shock peptide elicitor levels
Recognizing pathogen-associated molecular patterns on the cell surface is crucial for plant immunity. The proteinaceous nature of many of these patterns suggests that secreted proteases play important roles in their formation and stability. Here we demonstrate that the apoplastic subtilase SBT5.2a inactivates the immunogenicity of cold-shock proteins (CSPs) of the bacterial plant pathogen Pseudomonas syringae by cleaving within the immunogenic csp22 epitope. Consequently, mutant plants lacking SBT5.2a activity retain higher levels of csp22, leading to enhanced immune responses and reduced pathogen growth. SBT5.2 sensitivity is influenced by sequence variation surrounding the cleavage site and probably extends to CSPs from other bacterial species. These findings suggest that variations in csp22 stability among bacterial pathogens are a crucial factor in plant–bacteria interactions and that pathogens exploit plant proteases to avoid pattern recognition. Secreted plant subtilase SBT5.2 inactivates immunogenic csp22 epitopes in cold-shock proteins of Pseudomonas syringae, suggesting that pathogens exploit plant proteases to evade detection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


