二氧化碳电催化的时空活性相演变
IF 35.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
揭示和重定向高效电化学二氧化碳还原 (ECR) 催化剂中活性物种的动态演化具有挑战性。通过操作软 X 射线显微镜在纳米尺度上观察高效 ECR 催化剂的化学和形态演变,我们确定了阳离子铜物种在运行过程中的动态转变。在 Cu(OH)2 的动态相变过程中观察到的表面 Cu2+ 相进一步提高了 C-C 耦合活性,超出了金属铜相的能力。我们认为,碳酸氢氧化铜(II)物种可在阴极环境下动态存在。通过观察相变过程中的 Cu+ 相以及表面 Cu2+ 物种的形成,我们能够通过电化学方法将低活性催化剂重新定向为高活性催化剂,用于 C-C 偶联。密度泛函理论计算也支持 Cu2+ 物种可通过∗CO 吸附能量调制促进 C-C 偶联活性的提高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
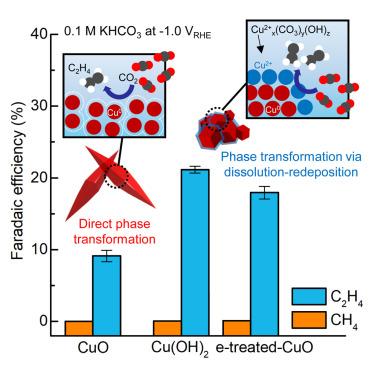
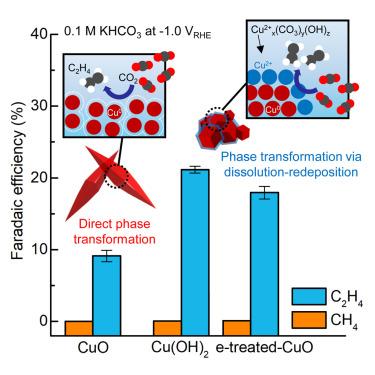
Spatiotemporal active phase evolution for CO2 electrocatalysis
Revealing and redirecting the dynamic evolution of active species in efficient electrochemical CO2 reduction (ECR) catalysts is challenging. By observing the chemical and morphological evolution in highly efficient ECR catalysts at the nanoscale via operando soft X-ray microscopy, we identified the dynamic transformation of cationic Cu species during operation. The surface Cu2+ phases, observed during a dynamic phase transformation of Cu(OH)2, additionally boost C–C coupling activity beyond the capability of metallic Cu phases. We suggest that copper(II)-carbonate-hydroxide species could dynamically persist under a cathodic environment. By observing the Cu+ phase during the phase transformation and the formation of surface Cu2+ species, we were able to electrochemically redirect low-active catalysts into high-active catalysts for C–C coupling. Density functional theory calculations also support that Cu2+ species could contribute to C–C coupling activity enhancement via ∗CO adsorption energy modulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


