人类 GBP1 装配抗菌膜衣的结构基础
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
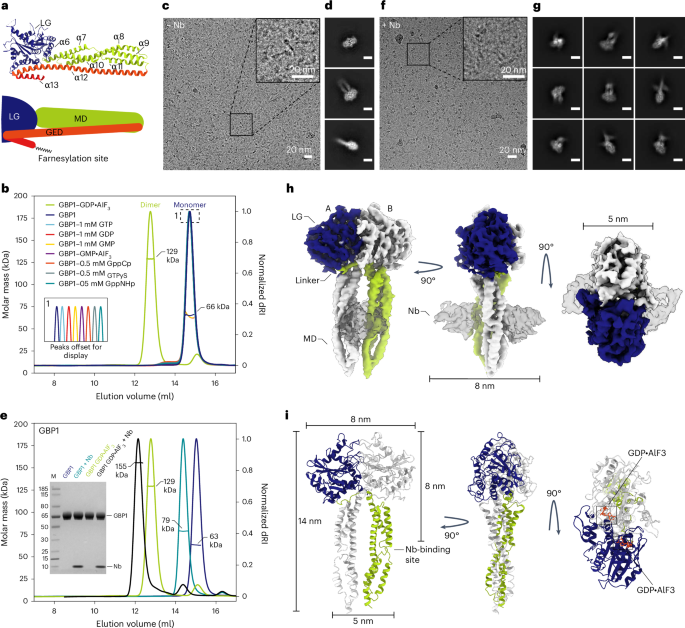
鸟苷酸结合蛋白(GBPs)是干扰素诱导的三磷酸鸟苷水解酶(GTPases),介导宿主对细胞内病原体的防御。它们的抗菌活性取决于其自我结合和包被病原体相关区室或细胞膜细菌的能力。包膜的形成依赖于 GTPase 的活性,但核苷酸的结合和水解如何促进包膜的形成仍不清楚。在这里,我们报告了全长人类 GBP1 二聚体在鸟嘌呤核苷酸结合状态下的冷冻电镜结构,并描述了脂质体和细菌脂多糖膜上 GBP1 涂层的分子超微结构。中间域和 GTPase 效应域的构象变化暴露了异戊烯化的 C 末端,使其与膜结合。α-螺旋中间结构域形成平行交叉排列,对包被的形成至关重要,并使延伸的效应结构域位于插入革兰氏阴性菌膜脂多糖层的位置。核苷酸的结合和水解产生了具有收缩能力的寡聚支架,促进了膜的挤压和破碎。我们的数据为了解 GBP1 在细胞内免疫中的效应功能提供了一个结构和机制框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Structural basis of antimicrobial membrane coat assembly by human GBP1
Guanylate-binding proteins (GBPs) are interferon-inducible guanosine triphosphate hydrolases (GTPases) mediating host defense against intracellular pathogens. Their antimicrobial activity hinges on their ability to self-associate and coat pathogen-associated compartments or cytosolic bacteria. Coat formation depends on GTPase activity but how nucleotide binding and hydrolysis prime coat formation remains unclear. Here, we report the cryo-electron microscopy structure of the full-length human GBP1 dimer in its guanine nucleotide-bound state and describe the molecular ultrastructure of the GBP1 coat on liposomes and bacterial lipopolysaccharide membranes. Conformational changes of the middle and GTPase effector domains expose the isoprenylated C terminus for membrane association. The α-helical middle domains form a parallel, crossover arrangement essential for coat formation and position the extended effector domain for intercalation into the lipopolysaccharide layer of gram-negative membranes. Nucleotide binding and hydrolysis create oligomeric scaffolds with contractile abilities that promote membrane extrusion and fragmentation. Our data offer a structural and mechanistic framework for understanding GBP1 effector functions in intracellular immunity. Kuhm et al. reveal how human guanylate-binding protein 1 (GBP1) dimers self-associate to coat bacterial pathogens and uncover a guanosine triphosphate hydrolase-dependent membrane-remodeling activity of GBP1 that is crucial for intracellular immunity
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


