用于近单细胞癌症成像的超亮近红外荧光 DNA 框架
IF 32.3
1区 物理与天体物理
Q1 OPTICS
引用次数: 0
摘要
接近单细胞水平的癌症成像对于研究体内细胞迁移和癌症转移非常理想。然而,目前的成像探针很难同时实现高灵敏度、深层组织穿透性和组织特异性。在此,我们报告了在第二个近红外窗口(1000-1700 nm,NIR-II)具有尾发射的尺寸和形状分辨荧光 DNA 框架(FDF)点,它能在肿瘤小鼠模型中实现近单细胞水平、肿瘤靶向深组织(约 1 cm)NIR-II 成像。通过构建内嵌疏水性纳米空腔的 DNA 框架,可非共价地封装设计的近红外-Ib(900-1000 纳米)探针(染料 Sq964)。FDF 点阵具有很高的水溶性、亮度和光稳定性。我们发现,FDF点能在肿瘤细胞中稳定存留并增强信号强度,是因为它们在肿瘤细胞中的积聚与形状有关。基于 FDF 点阵的癌症成像显示了低至 ~40 个肿瘤细胞的体内灵敏度、高至 ~26 的肿瘤与正常组织比率以及超过 11 天的长期成像。我们还展示了近红外-II成像引导的乳腺癌手术,可完全切除最小尺寸约为53微米(约20个细胞)的转移瘤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
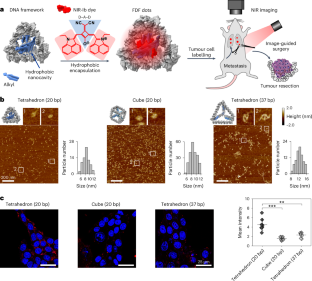

Ultrabright near-infrared fluorescent DNA frameworks for near-single-cell cancer imaging
Cancer imaging approaching single-cell levels is highly desirable for studying in vivo cell migration and cancer metastasis. However, current imaging probes struggle to simultaneously achieve high sensitivity, deep-tissue penetration and tissue specificity. Here we report size- and shape-resolved fluorescent DNA framework (FDF) dots with tail emission in the second near-infrared window (1,000–1,700 nm, NIR-II), which enable near-single-cell-level, tumour-targeting deep-tissue (~1 cm) NIR-II imaging in tumour-bearing mouse models. The construction of DNA frameworks with embedded hydrophobic nanocavity results in the non-covalent encapsulation of a designed NIR-Ib (900–1,000 nm) probe (dye Sq964). The FDF dots exhibit high water solubility, brightness and photostability. We find that the stable tumour retention of FDF dots with enhanced signal intensity arises from their shape-dependent accumulation in tumour cells. FDF-dot-based cancer imaging reveals in vivo sensitivity down to ~40 tumour cells, high tumour-to-normal tissue ratios up to ~26 and long-term imaging over 11 days. We also demonstrate NIR-II-image-guided breast cancer surgery with the complete excision of metastases with a minimum size of ~53 μm (~20 cells). Fluorescent DNA framework dots, consisting of a hydrophobic nanocavity containing a near-infrared-emitting dye, enable precise tumour imaging with sensitivity down to a few tens of cells in mouse models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Photonics
物理-光学
CiteScore
54.20
自引率
1.70%
发文量
158
审稿时长
12 months
期刊介绍:
Nature Photonics is a monthly journal dedicated to the scientific study and application of light, known as Photonics. It publishes top-quality, peer-reviewed research across all areas of light generation, manipulation, and detection.
The journal encompasses research into the fundamental properties of light and its interactions with matter, as well as the latest developments in optoelectronic devices and emerging photonics applications. Topics covered include lasers, LEDs, imaging, detectors, optoelectronic devices, quantum optics, biophotonics, optical data storage, spectroscopy, fiber optics, solar energy, displays, terahertz technology, nonlinear optics, plasmonics, nanophotonics, and X-rays.
In addition to research papers and review articles summarizing scientific findings in optoelectronics, Nature Photonics also features News and Views pieces and research highlights. It uniquely includes articles on the business aspects of the industry, such as technology commercialization and market analysis, offering a comprehensive perspective on the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


