促进成骨细胞分化和骨修复:光生物调节对脂肪基质细胞的启动效应
IF 3.7
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Journal of photochemistry and photobiology. B, Biology
Pub Date : 2024-10-02
DOI:10.1016/j.jphotobiol.2024.113040
引用次数: 0
摘要
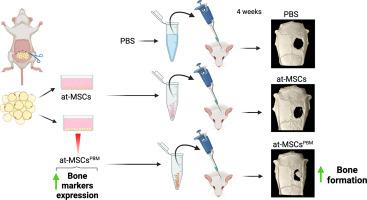
利用源自脂肪组织的间充质基质细胞(at-MSCs)治疗骨缺损的细胞疗法备受关注。因此,增强间充质干细胞成骨潜能的预处理策略可优化细胞疗法的结果,而光生物调控(PBM)疗法已成为一种有效、无创、低成本的替代方法。本研究探讨了光生物调控对at-MSCs分化的影响,以及细胞注射治疗后对骨缺损的修复。按照 PBM 方案(660 纳米;20 毫瓦;0.714 瓦/平方厘米;0.14 焦耳;5 焦耳/平方厘米)对大鼠 at-MSCs 进行培养和照射(at-MSCsPBM)。根据基因和蛋白质标记物的表达评估细胞分化情况。使用荧光检测活性氧(ROS)。将 At-MSCsPBM 注入 5 毫米长的腓骨病变部位,并通过显微 CT 和组织学评估分析骨形成情况。At-MSCs 用作对照。数据分析采用方差分析或 t 检验。At-MSCsPBM 表现出高水平的基因和蛋白runt相关转录因子-2(Runx2)和碱性磷酸酶(Alp)表达。PBM 提高了 ALP 活性并显著降低了 ROS 水平。此外,PBM 还增加了 Wnt 通路相关基因的表达。在体内,与对照组相比,经 at-MSCsPBM 处理的缺损的形态计量参数,包括骨量、骨量百分比、骨表面积和骨小梁数量均有所增加。这些研究结果表明,PBM能增强at-MSCs的成骨潜能,从而支持改善骨再生的细胞疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing osteoblast differentiation and bone repair: The priming effect of photobiomodulation on adipose stromal cells
Cellular therapy using adipose tissue–derived mesenchymal stromal cells (at-MSCs) has garnered attention for the treatment of bone defects. Therefore, preconditioning strategies to enhance the osteogenic potential of at-MSCs could optimize cell therapy outcomes, and photobiomodulation (PBM) therapy has emerged as an effective, noninvasive, and low-cost alternative. This study explored the impacts of PBM on at-MSCs differentiation and the subsequent repair of bone defects treated with cell injection. Rat at-MSCs were cultured and irradiated (at-MSCsPBM) following the PBM protocol (660 nm; 20 mW; 0.714 W/cm2; 0.14 J; 5 J/cm2). Cellular differentiation was assessed based on the expression of gene and protein markers. Reactive oxygen species (ROS) were detected using fluorescence. At-MSCsPBM were injected into 5-mm calvarial lesions, and bone formation was analyzed using micro-CT and histological evaluations. At-MSCs were used as control. Data were analyzed using the ANOVA or t-test. At-MSCsPBM exhibited high levels of gene and protein runt-related transcription factor-2 (Runx2) and alkaline phosphatase (Alp) expression. PBM increased ALP activity and significantly reduced ROS levels. In addition, PBM increased the expression of Wnt pathway–associated genes. In vivo, there was an increase in the morphometric parameters, including bone volume, percentage of bone volume, bone surface area, and trabecular number, in at-MSCsPBM-treated defects compared with those in the control. These findings suggest that PBM enhances the osteogenic potential of at-MSCs, thereby supporting the advancement of improved cellular therapies for bone regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.10
自引率
1.90%
发文量
161
审稿时长
37 days
期刊介绍:
The Journal of Photochemistry and Photobiology B: Biology provides a forum for the publication of papers relating to the various aspects of photobiology, as well as a means for communication in this multidisciplinary field.
The scope includes:
- Bioluminescence
- Chronobiology
- DNA repair
- Environmental photobiology
- Nanotechnology in photobiology
- Photocarcinogenesis
- Photochemistry of biomolecules
- Photodynamic therapy
- Photomedicine
- Photomorphogenesis
- Photomovement
- Photoreception
- Photosensitization
- Photosynthesis
- Phototechnology
- Spectroscopy of biological systems
- UV and visible radiation effects and vision.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


