LncRNA6524/miR-92a-2-5p/Dvl1/Wnt/β-catenin轴促进了UUO小鼠模型的肾脏纤维化。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
据报道,LncRNA 参与多种生物和病理过程,包括阻塞性肾病导致的肾脏纤维化。然而,每种lncRNA在这方面的功能和机制各不相同。在这项研究中,我们通过TGF-β1处理在体外建立了一个肾脏纤维化模型,并通过单侧输尿管梗阻在体内建立了一个肾脏纤维化模型。经 qPCR 证实,两种模型中 lncRNA6524 的表达均有所增加。此外,我们还发现lncRNA6524介导了TGF-β1诱导的细胞外基质(ECM)蛋白在BUMPT细胞中的积累。我们使用双荧光素酶报告实验、免疫荧光和 qPCR 研究了这一机制。我们的研究结果表明,lncRNA6524 可作为 miR-92a-2-5p 的海绵,通过上调 Dvl1/Wnt/β-catenin 信号通路促进肾脏纤维化。总之,我们的研究结果表明,在肾梗阻过程中,肾上皮细胞中的 lncRNA6524、miR-92a-2-5p 和 Dvl1/Wnt/β-catenin 轴之间存在线性调控关系。这为治疗梗阻相关的肾脏纤维化提供了一个新的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
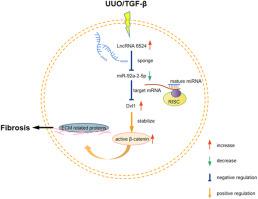
The LncRNA6524/miR-92a-2-5p/Dvl1/Wnt/β-catenin axis promotes renal fibrosis in the UUO mouse model
LncRNAs are reported to participate in multiple biological and pathological processes, including renal fibrosis due to obstructive nephropathy. However, the function and mechanisms of each lncRNA in this context differ. In this study, we created a fibrosis model in vitro using TGF-β1 treatment and in vivo through unilateral ureteral obstruction. We demonstrated that lncRNA6524 expression increased in both models, as confirmed by qPCR. Additionally, we discovered that lncRNA6524 mediates the TGF-β1-induced accumulation of extracellular matrix (ECM) proteins in BUMPT cells. We investigated the mechanism using dual luciferase reporter assays, immunofluorescence, and qPCR. Our results indicate that lncRNA6524 acts as a sponge for miR-92a-2-5p, promoting renal fibrosis by upregulating the Dvl1/Wnt/β-catenin signaling pathway. In summary, our findings demonstrate a linear regulatory relationship among lncRNA6524, miR-92a-2-5p, and the Dvl1/Wnt/β-catenin axis in renal epithelial cells during kidney obstruction. This highlights a new potential target for treating obstruction-related renal fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


