双核定位序列是 ADARa 在沙蚕体内进行核导入和稳定自我嵌合所不可或缺的。
IF 3.2
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在哺乳动物和果蝇中,保守的转录后修饰是双链 RNA 中的腺苷-肌苷(A-to-I)脱氨,由称为作用于 RNA 的腺苷脱氨酶(ADARs)的 RNA 编辑酶催化。ADARs 的传统核导入途径包括导入蛋白 α4 和 α5 识别推测的经典核定位序列(NLS)。在我们之前的研究中,证实了家蚕中的 ADAR(BmADARa)主要位于细胞核中。然而,BmADARa 中 NLS 的位置及其对核导入和自二聚化的影响仍不清楚。利用 NLS 预测软件,我们预测在 BmADARa 的氨基末端 85 个氨基酸(N85)中存在一个双侧 NLS。我们通过点突变验证了这一预测,结果表明双端 NLS 可直接介导 BmADARa 的核导入。共免疫沉淀分析表明,BmADARa的核导入主要依赖于BmKaryopherin α3(与哺乳动物的导入蛋白α4同源),尽管BmKaryopherin α3和BmImportin α5都能识别双端NLS。BmADARa的N-端截短突变体和双端NLS突变体表明,双端NLS是BmADARa的主要核导入位点和自嵌合的关键结构。总之,BmADARa的N端双端NLS被BmKaryopherin α3和BmImportin α5识别,促进了其核导入。这促进了 BmADARa 的自二聚化并保持了二聚化的稳定性,从而提高了其对目标底物的编辑效率。该研究结果证明了双侧 NLS 在 BmADARa 编辑过程中的作用,为进一步研究 BmADARa 在森蛙生长发育过程中的调控作用奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
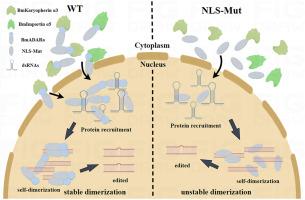
Bipartite nuclear localization sequence is indispensable for nuclear import and stability of self-dimerization of ADARa in Bombyx mori
The conservative post-transcriptional modification in mammals and Drosophila is adenosine-to-inosine (A-to-I) deamination in double-stranded RNA, catalyzed by RNA-editing enzymes known as adenosine deaminases acting on RNA (ADARs). The traditional nuclear import pathway for ADARs involves the recognition of a putative classical nuclear localization sequence (NLS) by importin α4 and α5. In our previous research, ADAR in silkworm, Bombyx mori (BmADARa) was confirmed predominantly located in the nucleus. However, the location of the NLS in BmADARa and its impact on nuclear import and self-dimerization remained unclear. Utilizing NLS prediction software, we predicted the presence of a bipartite NLS within the amino-terminal, 85 amino acids of BmADARa (N85). This prediction was validated through point mutation, which demonstrated that the bipartite NLS could directly mediate nuclear import of BmADARa. Co-immunoprecipitation analysis revealed that BmADARa is mainly dependent on BmKaryopherin α3 (homologous to mammalian importin α4) for nuclear import, although both BmKaryopherin α3 and BmImportin α5 could recognize bipartite NLS. The N-terminal truncated mutants and the bipartite NLS mutants of BmADARa suggest that the bipartite NLS is the major nuclear import site and a crucial structure for self-dimerization of BmADARa. In conclusion, the N-terminal bipartite NLS of BmADARa is recognized by BmKaryopherin α3 and BmImportin α5, facilitating its nuclear import. This promotes BmADARa self-dimerization and maintains the stability of dimerization, thereby enhancing its editing efficiency on target substrates. The results of this research demonstrate the role of bipartite NLS in BmADARa editing and laying a foundation for further research on the regulation of BmADARa in the growth and development in B. mori.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


