P-Stereodefined morpholino dinucleoside 3',5'-phosphorothioates.
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们首次采用改良的 1,3,2-oxathiaphospholane 法(OTP 法)合成了 P-stereodefined morpholino phosphorothioate 类似物,并就其立体化学提供了宝贵的结构见解。合成了吗啉型核苷(mU-OTPs)的 N-(2-硫代-4,4-五亚甲基-1,3,2-氧硫磷杂环戊烷)衍生物,并将其分离成纯净的 P-非对映异构体,用于制备 P-立体化学定义的吗啉二核苷 3',5'-硫代磷酸酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
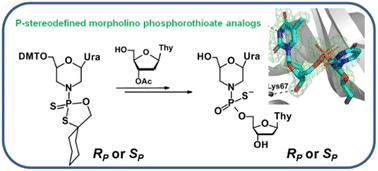
P-Stereodefined morpholino dinucleoside 3′,5′-phosphorothioates†
Here, we present for the first time the synthesis of P-stereodefined morpholino phosphorothioate analogs by using a modified 1,3,2-oxathiaphospholane method (OTP method) and provide valuable structural insights into their stereochemistry. N-(2-Thio-4,4-pentamethylene-1,3,2-oxathiaphospholane) derivatives of morpholino-type nucleosides (mU-OTPs) were synthesized, separated into pure P-diastereomers and used to prepare P-stereodefined morpholino dinucleoside 3′,5′-phosphorothioates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


