手性磷酸催化的 3,4-二氢异喹啉与 1-萘酚的不对称 Aza Friedel-Crafts 反应。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-01
Epub Date: 2024-10-10
DOI:10.1021/acs.joc.4c01617
引用次数: 0
摘要
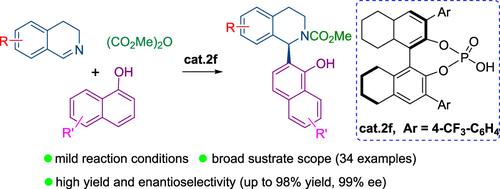
介绍了一种高效的手性磷酸催化的 3,4-二氢异喹啉和 1-萘酚的不对称氮杂 Friedel-Crafts 反应。该反应为在 C1 位合成具有 1-萘酚取代基的多种手性四氢异喹啉提供了一种通用方法,并具有极佳的产率和对映选择性。根据所进行的机理实验,提出了一种合理的催化机理。此外,该反应在克级规模上的应用也成功证明了其实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Asymmetric Aza Friedel-Crafts Reaction of 3,4-Dihydroisoquinolines with 1-Naphthols Catalyzed by Chiral Phosphoric Acids.
An efficient chiral phosphoric acid-catalyzed asymmetric aza Friedel-Crafts reaction of 3,4-dihydroisoquinolines and 1-naphthols is described. The reaction provides a general method for the synthesis of diverse chiral tetrahydroisoquinoline with 1-naphthol substituents at the C1-position in excellent yields and enantioselectivities. Based on the conducted mechanistic experiments, a plausible catalytic mechanism was proposed. Moreover, the practicability of the reaction is successfully demonstrated by its application on a gram scale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


