以水为氧源的逐步电化学拜尔-维利格氧化法
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Baeyer-Villiger 氧化法是一种具有 125 年历史的方法,它通过协同机制与按比例配比的过酸反应生成内酯。因此,取代的内酯只能从取代的环酮中获得。在此背景下,我们利用 CeO2@PbO2@Ti 电极开发了一种电化学拜耳-维里格氧化法,该方法通过逐步机制产生取代内酯。PbO2 与苯甲酸分子催化剂结合,可以产生并利用电化学水分裂产生的活性氧作为氧化剂。CeO2 旨在促进分步机制,同时抑制协同机制。因此,可以通过碳位重排过程,以高选择性(77%)和高产率(20 mM)从未予取代的环酮生产取代的内酯。所开发的以水为氧源的逐步电化学拜耳-维利格氧化法为有机合成提供了一种新的绿色方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
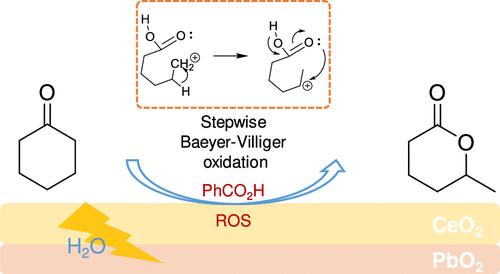
A Stepwise Electrochemical Baeyer–Villiger Oxidation with Water as the Oxygen Source
Baeyer–Villiger oxidation is a method with a 125-year history that produces lactones through a synergistic mechanism by reaction with stoichiometric peracids. Therefore, substituted lactones can be obtained from only substituted cyclic ketones. In this context, an electrochemical Baeyer–Villiger oxidation was developed using a CeO2@PbO2@Ti electrode, which produces substituted lactones through a stepwise mechanism. PbO2, in combination with a benzoic acid molecular catalyst, can generate and utilize reactive oxygen species from electrochemical water splitting to serve as the oxidant. CeO2 is designed to promote the stepwise mechanism while suppressing the synergistic mechanism. Therefore, substituted lactone can be produced from unsubstituted cyclic ketone with high selectivity (77%) and yield (20 mM) through a carbocation rearrangement process. The developed stepwise electrochemical Baeyer–Villiger oxidation, using water as the oxygen source, offers a new green approach to organic synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


