CMP 伪氨基酸合成酶 PseF 的结构剖析
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
假酰胺酸是一种非哺乳动物糖,存在于包括几种人类病原体在内的许多细菌的表面糖共轭物中,是一种被认为有助于逃避免疫的毒力因子。该糖核苷酸活化形式 CMP-Pse5Ac7Ac 生物合成的最后一步由 CMP-Pse5Ac7Ac 合成酶(PseF)完成。在这里,我们介绍了来自鱼腥单胞菌(Aeromonas caviae)的 PseF(AcPseF)的生物化学和结构特征,AcPseF 在广泛的 pH 值和温度范围内显示出依赖金属的活性。与 CMP-Pse5Ac7Ac 结合后,AcPseF 与其他 CMP- 乌洛托品酸合成酶一样发生动态运动。通过与侧链官能团的活性位点相互作用以及将分子定位在疏水袋中,该酶可以清楚地将 Pse5Ac7Ac 与其他乌洛托品酸区分开来。最后,我们发现 AcPseF 以能量最低的构象结合 CMP-Pse5Ac7Ac 侧链,我们在该类其它酶的结构中也观察到了这种趋势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
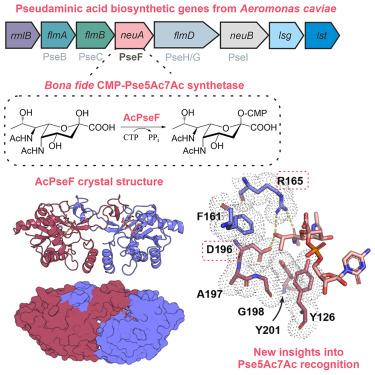
Structural dissection of the CMP-pseudaminic acid synthetase, PseF
Pseudaminic acid is a non-mammalian sugar found in the surface glycoconjugates of many bacteria, including several human pathogens, and is a virulence factor thought to facilitate immune evasion. The final step in the biosynthesis of the nucleotide activated form of the sugar, CMP-Pse5Ac7Ac is performed by a CMP-Pse5Ac7Ac synthetase (PseF). Here we present the biochemical and structural characterization of PseF from Aeromonas caviae (AcPseF), with AcPseF displaying metal-dependent activity over a broad pH and temperature range. Upon binding to CMP-Pse5Ac7Ac, AcPseF undergoes dynamic movements akin to other CMP-ulosonic acid synthetases. The enzyme clearly discriminates Pse5Ac7Ac from other ulosonic acids, through active site interactions with side-chain functional groups and by positioning the molecule in a hydrophobic pocket. Finally, we show that AcPseF binds the CMP-Pse5Ac7Ac side chain in the lowest energy conformation, a trend that we observed in the structures of other enzymes of this class.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


