共价有机框架衍生的 B/N 共掺杂碳 FLPs 用于将 α、β-不饱和醛选择性加氢转化为不饱和醇的无金属催化剂
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
目前的一项挑战是如何精确定制受挫路易斯对(FLPs)位点,以构建全固态 FLPs 无金属催化剂,使其在活化 H2 方面与同质/金属催化剂一样有效,甚至更有效。本研究采用配体交换策略和自模板碳化法,在富氮共价有机框架(SNW-1)中有针对性地掺入 B 原子,从而精确地定制出 FLPs 位点,制备出 B/N 共掺碳(SNW-BCN)催化剂。催化剂随后被用于将 α、β-不饱和醛选择性氢化为不饱和醇。研究发现,从富氮的 SNW-1 中可以获得大量的吡啶-N 位点。此外,还可以通过引入带有路易斯酸杂原子的有机配体(4-甲酰基苯硼酸)来实现 B 原子的定向掺杂,从而抢占 SNW-1 的配体位点。此外,B 原子与邻近 N 原子在高温下优先形成的 B-N 共价键也可以作为路易斯酸位点。DFT 计算和原位表征表明,邻近的富电子吡啶-N 和缺电子 B-N 位点可形成 B-N/pyridinic-N 路易斯酸位点,能有效激活 H2 和 α、β-不饱和醛的 C═O,H-H 键的解离能仅为 0.36 eV。这项工作促进了不饱和醇的环保型合成,并为开发和合成全固态 FLPs 无金属催化剂提供了新的概念。此外,还对催化剂放大实验进行了研究,因为这些实验可能会为催化剂的大规模生产提供启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
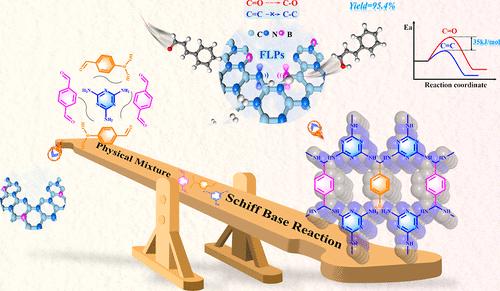
Covalent Organic Framework-Derived B/N Co-Doped Carbon FLPs Metal-Free Catalysts for the Selective Hydrogenation of α,β-Unsaturated Aldehydes to Unsaturated Alcohols
An ongoing challenge is to precisely tailor the frustrated Lewis pairs (FLPs) sites to construct all-solid-state FLPs metal-free catalysts that are as effective as or even more effective than homogeneous/metal catalysts in H2 activation. In this study, B/N codoped carbon (SNW-BCN) catalysts were prepared by precisely tailoring FLPs sites by targeted doping of B atoms in the nitrogen-rich covalent organic framework (SNW-1) using the ligand-exchange strategy and self-templated carbonization. The catalysts were then applied in the selective hydrogenation of α,β-unsaturated aldehydes to unsaturated alcohols. It was found that a significant amount of pyridinic-N sites could be obtained from nitrogen-rich SNW-1. Moreover, targeted doping of B atoms can be accomplished by introducing organic ligands (4-formylphenylboronic acid) with Lewis acid heteroatoms to pre-empt the ligand site of SNW-1. Additionally, the B–N covalent bond, which preferentially forms between B and neighboring N at high temperature, can function as a Lewis acid site. DFT calculations and in situ characterizations show that the neighboring electron-rich pyridinic-N and the electron-deficient B–N site can form B–N/pyridinic-N FLPs sites, which can effectively activate H2 and the C═O of α,β-unsaturated aldehydes, with only 0.36 eV of H–H bond dissociation energy. This work encourages the environmentally friendly synthesis of unsaturated alcohols and offers fresh concepts for the development and synthesis of all-solid-state FLPs metal-free catalysts. Additionally, experiments on catalyst scale-up were also investigated because they might shed light on catalyst production on a large scale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


