钯催化苯并硅杂环丁烯的 Si-C (sp3) 键双活化与意想不到的烯烃迁移和开环水解协同作用
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
硅碳键的区域选择性活化已成为获得带有官能团的有机硅化合物的有力策略。相比之下,用于串联和双硅碳键活化硅圈的新反应体系的开发却进展缓慢。在这方面,还没有关于苯并硅环丁烯的 Si-C(sp3)键活化及其串联转化的报道。在本文中,我们针对这一难题,首次公开了钯催化苯并硅杂环丁烯的 Si-C 双键活化及其串联扩环和开环转化的实例,在该反应中合成了各种具有酯基的官能化硅烷醇。这种新型转化的主要特点是高选择性地活化苯并硅烷的 Si-C(sp3)键,从而与酯活化的炔烃实现[4 + 2]环加成,随后与 H2O 进行开环和σ键元合成。此外,DFT 研究还揭示了 Si-C (sp3) 键活化、烯烃迁移和开环水解在未预见到的反应过程中的起源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
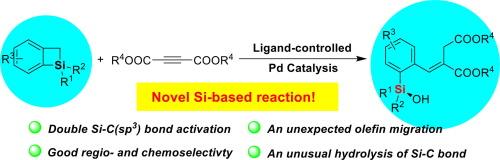
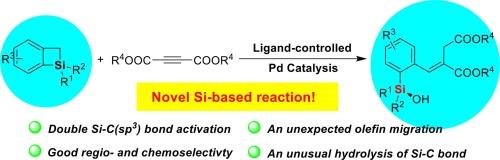
Palladium-catalyzed double activation of Si-C(sp3) bond of benzosilacyclobutenes synergized with unexpected olefin migration and ring-opening hydrolysis
A regioselective silicon-carbon bond activation of silacycles has emerged as a powerful strategy to access organosilicon compounds with functional groups. In contrast, progress in the development of new reaction systems for the tandem and double Si-C bond activation of silacycles has lagged. In this regard, there have been no reports of Si-C(sp3) bond activation of benzosilacyclobutenes and its tandem transformations. Herein, we address this challenging, disclosing the first example of palladium-catalyzed double Si-C bond activation of benzosilacyclobutenes and its tandem ring expansion and ring-opening transformations, in which various functionalized silanols that have ester groups are synthesized in this reaction. The main feature of this novel transformation is the highly selective activation of the Si-C(sp3) bond of benzosilacyles to achieve [4 + 2] cycloaddition with ester-activated alkynes and subsequent ring-opening and σ-bond metathesis with H2O. Moreover, the DFT studies realized the origin of Si-C(sp3) bond activation, olefin migration, and ring-opening hydrolysis in the unprecedent reaction process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


