实现雌二醇水平的无创测定:开发和验证用于定量唾液中亚克/毫升水平雌二醇的 LC-MS/MS 检测方法
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
背景雌二醇(E2)是一种女性性激素,参与多种生物过程。尽管雌二醇水平通常通过血液样本进行测量,但使用非侵入性技术(如测定唾液中的雌二醇)可重复采集样本,并纳入更多的参与者。测量唾液 E2 的免疫测定技术无法准确反映月经周期中观察到的 E2 血浆浓度变化,这可能是由于所需的灵敏度较高(在亚皮克/毫升范围内)。因此,我们需要灵敏、耐用的分析方法来测定唾液中的 E2。结果该方法基于 1,2-二甲基-1H-咪唑-5-磺酰氯的化学衍生和液相色谱-串联质谱分析,通过对高特异性 SRM 过渡进行求和。这种方法提高了灵敏度。该方法的验证结果表明,即使在 250 fg/mL 的浓度下,1 mL 唾液中 E2 的定量也是准确和精确的(日内准确度为 97%,RSD 为 15%;日间准确度为 104%,RSD 为 18%)。为了评估其功效,我们分析了 5 名健康女性志愿者在整个月经周期中采集的唾液样本。分析结果表明,测量样本中 E2 浓度的变化反映了完整月经周期中的预期变化。据我们所知,这是第一种可以精确测量健康女性整个月经周期唾液样本中 E2 含量的方法。它也适用于孕期雌二醇的测定。它的高灵敏度使其成为评估激素分泌在女性健康中的作用的理想方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
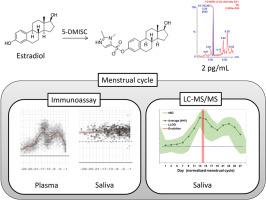
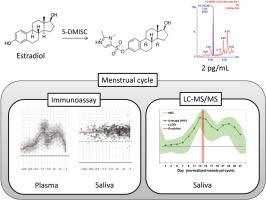
Towards the non-invasive determination of estradiol levels: Development and validation of an LC-MS/MS assay for quantification of salivary estradiol at sub-pg/mL level
Background
Estradiol (E2) is a female sex hormone involved in several biological processes. Although E2 levels are commonly measured in blood samples, the use of non-invasive techniques (e.g. determination of salivary E2) would allow for the collection of repeated samples and the inclusion of a greater number of participants. Immunoassay-based techniques to measure salivary E2 failed to accurately mirror the variations observed in the plasmatic concentrations of E2 during the menstrual cycle probably due to the high sensitivity required (in the sub-pg/mL range). Therefore, sensitive and rugged analytical methods for the determination of salivary E2 are required. For this, we developed and validated an analytical methodology for the accurate determination of salivary E2.
Results
The method is based on chemical derivatization with 1,2-dimethyl-1H-imidazole-5-sulphonyl chloride and liquid chromatography-tandem mass spectrometry analysis by summing highly-specific SRM transitions. This strategy allowed for increasing the sensitivity of the method. The validation of the method showed an accurate and precise quantification of E2 in 1 mL of saliva even at 250 fg/mL (97 % accuracy and 15 % RSD intra-day, and 104 % accuracy and 18 % RSD inter-day). In order to evaluate its efficacy, we analysed saliva samples from 5 healthy female volunteers collected during a whole menstrual cycle. Our analyses showed that the variations in the concentration of E2 in the measured samples mirrored those expected during a complete menstrual cycle. Additionally, we validated the suitability of our method for determining salivary E2 levels during pregnancy.
Significance
To the best of our knowledge, this is the first method that allows to precisely and accurately measuring E2 in saliva samples along the whole menstrual cycle of healthy females. It is also suitable for the determination of estradiol during pregnancy. Its high sensitivity makes this strategy ideal for the evaluation of the role of hormone production in women's health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


