通过共聚焦光场显微镜对小鼠大脑中的神经元群进行体积电压成像。
IF 36.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
电压成像可直接测量神经元的活动,有望了解单个神经元和整个神经元群的信息处理过程。然而,由于同时需要较高的成像速度和信噪比、较大的体积覆盖范围和较低的光漂白率,对大量神经元群进行电压成像一直是一项挑战。为了克服这一挑战,我们开发了一种共聚焦光场显微镜,通过采用速度增强型相机、快速稳健的扫描机制、激光斑点噪声消除和优化的光效,在速度和噪声性能方面超越了传统的限制。利用这种方法,我们在小鼠皮层直径 800 微米、厚 180 微米的体积内同时记录了 300 多个尖峰神经元,时间超过 20 分钟。通过整合空间和电压活动曲线,我们绘制出了清醒小鼠大脑的三维神经协调模式图。我们的方法对于体积电压成像的常规应用非常稳健。本文章由计算机程序翻译,如有差异,请以英文原文为准。
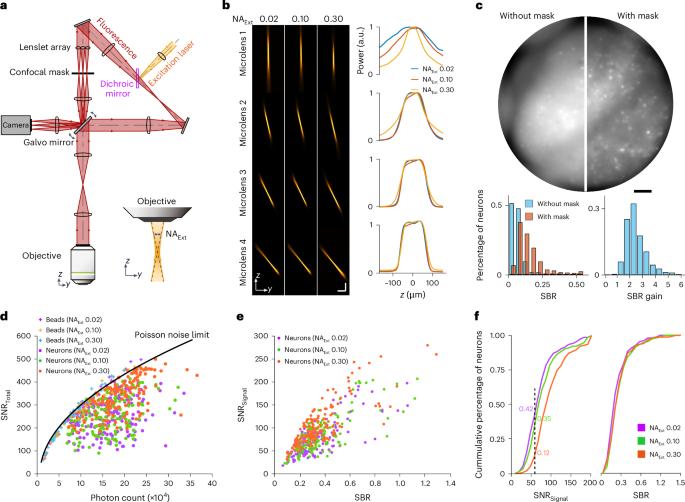
Volumetric voltage imaging of neuronal populations in the mouse brain by confocal light-field microscopy
Voltage imaging measures neuronal activity directly and holds promise for understanding information processing within individual neurons and across populations. However, imaging voltage over large neuronal populations has been challenging owing to the simultaneous requirements of high imaging speed and signal-to-noise ratio, large volume coverage and low photobleaching rate. Here, to overcome this challenge, we developed a confocal light-field microscope that surpassed the traditional limits in speed and noise performance by incorporating a speed-enhanced camera, a fast and robust scanning mechanism, laser-speckle-noise elimination and optimized light efficiency. With this method, we achieved simultaneous recording from more than 300 spiking neurons within an 800-µm-diameter and 180-µm-thick volume in the mouse cortex, for more than 20 min. By integrating the spatial and voltage activity profiles, we have mapped three-dimensional neural coordination patterns in awake mouse brains. Our method is robust for routine application in volumetric voltage imaging. Confocal light-field microscopy allows volumetric voltage imaging in the mouse brain at speeds sufficiently high to extract the spiking activity of hundreds of neurons.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Methods
生物-生化研究方法
CiteScore
58.70
自引率
1.70%
发文量
326
审稿时长
1 months
期刊介绍:
Nature Methods is a monthly journal that focuses on publishing innovative methods and substantial enhancements to fundamental life sciences research techniques. Geared towards a diverse, interdisciplinary readership of researchers in academia and industry engaged in laboratory work, the journal offers new tools for research and emphasizes the immediate practical significance of the featured work. It publishes primary research papers and reviews recent technical and methodological advancements, with a particular interest in primary methods papers relevant to the biological and biomedical sciences. This includes methods rooted in chemistry with practical applications for studying biological problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


