生物制药缓冲体系中聚山梨醇酯 80 稳定性差异的比较分析和机理研究。
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-09
DOI:10.1016/j.ejpb.2024.114521
引用次数: 0
摘要
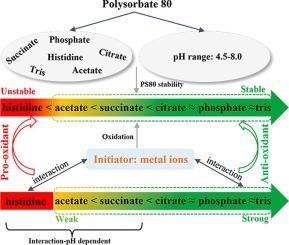
聚山梨醇酯 80(PS80)是一种非离子表面活性剂,广泛用于生物制药配方中稳定蛋白质。然而,在过去十年中,PS80 降解已成为业界普遍关注的问题。本研究评估了最常用的 pH 值/缓冲体系对 PS80 稳定性的影响。PS80 在组氨酸缓冲液中降解最快,其次是醋酸盐和琥珀酸缓冲液,而在柠檬酸盐、磷酸盐和三羟甲基氨基甲烷缓冲液中则保持稳定。当 PS80 发生降解时,降解程度与 pH 值有关。主要的降解途径是由金属离子引发的氧化。PS80 在不同 pH 值/缓冲液体系中具有不同的稳定性,这归因于缓冲剂的作用,缓冲剂可通过与金属离子的相互作用促进或抑制氧化过程。具体来说,除组氨酸外,其他缓冲剂都表现出与乙二胺四乙酸(EDTA)类似的金属离子螯合作用,从而抑制了 PS80 的氧化,但螯合作用的程度各不相同。此外,在醋酸盐和琥珀酸缓冲液中,pH 值越高,结合能力越强。相反,据报道组氨酸会与金属离子形成促氧化复合物,从而加速 PS80 的降解,尤其是在较高的 pH 值水平下。我们的研究首次全面了解了 PS80 在生物制药缓冲体系中的氧化作用。这为肠外制剂中缓冲剂和辅料的选择奠定了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comparative analysis and mechanistic insights into polysorbate 80 stability differences in biopharmaceutical buffer systems
Polysorbate 80 (PS80) is a non-ionic surfactant extensively utilized in biopharmaceutical formulations for stabilizing proteins. However, PS80 degradation has become a widespread concern throughout the industry over the past decade. In this work, the impact of most frequently employed pH/buffer systems on the stability of PS80 was assessed. PS80 degraded fastest in histidine buffer, followed by acetate and succinate buffers, whereas it remained stable in citrate, phosphate and tris buffers. When there was PS80 degradation, the extent of degradation was found to be pH-dependent. The predominant degradation pathway was oxidation mainly triggered by metal ions. The varying stability of PS80 across different pH/buffer systems was attributed to the role of buffer agents, which can either promote or inhibit the oxidation process through their interactions with metal ions. Specifically, buffers except histidine exhibited metal ion chelation similar to ethylenediaminetetraacetic acid (EDTA), which can suppress the oxidation of PS80, although the effectiveness of chelation varies to different extents. Furthermore, the binding capacity appeared stronger at higher pH in acetate and succinate buffers. Conversely, histidine was reported to form pro-oxidant complexes with metal ions to accelerate PS80 degradation, especially at higher pH levels. Our work for the first time offers a comprehensive understanding of PS80 oxidation in biopharmaceutical buffer systems. This provides a strong foundation for buffer and excipient selection in parenteral formulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


