抑制 TREM-1 可通过 PI3K/AKT/FoxO3a 信号通路减轻巨噬细胞浸润和炎症,从而改善血管紧张素 II 诱导的心房颤动。
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
炎症和免疫细胞浸润与心房颤动(房颤)的发病机制密切相关。髓系细胞上表达的触发受体-1(TREM-1)是一种炎症增强因子,与多种心血管疾病有关。然而,TREM-1在心房颤动发病过程中的确切作用和潜在机制仍不明确。研究人员使用房颤患者的心房样本来评估 TREM-1 的表达水平。建立了血管紧张素II(Ang II)诱导的房颤小鼠模型,以评估TREM-1的功能。通过超声心动图、程序化经静脉心脏起搏和心房电生理图评估心脏功能和房颤诱导性。使用流式细胞术评估了外周血和心房炎症细胞。通过组织学、大量 RNA 测序、生化分析和细胞培养,阐明了 TREM-1 在房颤中的机制作用。在心房颤动患者的心房中,TREM-1表达上调并与巨噬细胞共定位。药物抑制 TREM-1 可减少 Ang II 诱导的心房扩大和电重塑。抑制 TREM-1 还能改善 Ang II 诱导的 NLRP3 炎症小体激活、炎症因子释放、心房纤维化和巨噬细胞浸润。转录组分析表明,TREM-1 通过 PI3K/AKT/FoxO3a 信号通路调节 Ang II 诱导的炎症。体外研究进一步证实了这些发现,表明TREM-1的激活会加剧Ang II诱导的炎症,而过量表达FoxO3a则会抵消这种效应。这项研究发现了 TREM-1 在心房颤动发病机制中的关键作用及其潜在的分子机制。抑制 TREM-1 为治疗心房颤动提供了一种新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
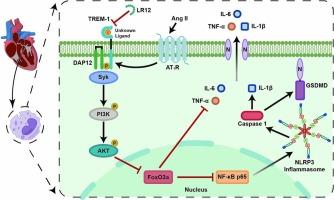
Inhibition of TREM-1 ameliorates angiotensin II-induced atrial fibrillation by attenuating macrophage infiltration and inflammation through the PI3K/AKT/FoxO3a signaling pathway
Inflammation and infiltration of immune cells are intricately linked to the pathogenesis of atrial fibrillation (AF). Triggering receptor expressed on myeloid cells-1 (TREM-1), an enhancer of inflammation, is implicated in various cardiovascular disorders. However, the precise role and potential mechanisms of TREM-1 in the development of AF remain ambiguous. Atrial samples from patients with AF were used to assess the expression levels of TREM-1. An angiotensin II (Ang II)-induced AF mouse model was established to assess the functionality of TREM-1. Cardiac function and AF inducibility were assessed through echocardiography, programmed transvenous cardiac pacing, and atrial electrophysiological mapping. Peripheral blood and atrial inflammatory cells were assessed using flow cytometry. Using histology, bulk RNA sequencing, biochemical analyses, and cell cultures, the mechanistic role of TREM-1 in AF was elucidated. TREM-1 expression was upregulated and co-localized with macrophages in the atria of patients with AF. Pharmacological inhibition of TREM-1 decreased Ang II-induced atrial enlargement and electrical remodeling. TREM-1 inhibition also ameliorated Ang II-induced NLRP3 inflammasome activation, inflammatory factor release, atrial fibrosis, and macrophage infiltration. Transcriptomic analysis revealed that TREM-1 modulates Ang II-induced inflammation through the PI3K/AKT/FoxO3a signaling pathway. In vitro studies further supported these findings, demonstrating that TREM-1 activation exacerbates Ang II-induced inflammation, while overexpression of FoxO3a counteracts this effect. This study discovered the critical role of TREM-1 in the pathogenesis of AF and its underlying molecular mechanisms. Inhibition of TREM-1 provides a new therapeutic strategy for the treatment of AF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


