介孔氧化铈纳米酶通过增强抗窒息性来有效抑制肿瘤和转移。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
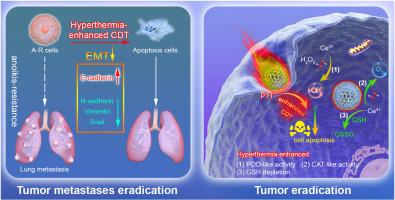
肿瘤细胞脱离细胞外基质或失去细胞间连接后仍能存活,这种现象被称为抗瘤性(AR)。AR与肿瘤细胞的转移和增殖密切相关,能使它们在脱离后扩散、迁移和入侵。在此,我们研究了一种由介孔二氧化硅/纳米氧化铈(MSN-Ce@SP/PEG)组成的新型复合纳米酶。这种纳米酶表现出令人满意的过氧化氢酶(CAT)活性,能有效地将肿瘤细胞内高浓度的H2O2转化为O2,从而有效缓解肿瘤缺氧。此外,MSN-Ce@SP/PEG纳米酶还表现出较高的过氧化物酶(POD)活性,可提高活性氧(ROS)水平,减轻肝细胞癌(HCC)细胞的AR。MSN-Ce@SP/PEG 纳米酶在 CAT 和 POD 方面表现出令人满意的双重生物活性,并在有利的光热条件下显著增强。通过这些能力的协同作用,该纳米酶破坏了离体 HCC 细胞的上皮-间质转化(EMT)过程,最终抑制了耐 anoikis HCC 细胞的复发和转移潜力。这项研究首次报道了一种基于介孔二氧化硅/纳米氧化铈的新型纳米酶,可用于治疗 HCC 细胞中的 AR,从而抑制 HCC 的复发和转移。这项工作的发现为开发预防 HCC 复发和转移的创新策略提供了开创性的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mesoporous cerium oxide nanoenzyme for Efficacious impeding tumor and metastasis via Conferring resistance to anoikis
Tumor cells can survive when detached from the extracellular matrix or lose cell-to-cell connections, leading to a phenomenon known as anoikis resistance (AR). AR is closely associated with the metastasis and proliferation of tumor cells, enabling them to disseminate, migrate, and invade after detachment. Here, we have investigated a novel composite nanoenzyme comprising mesoporous silica/nano-cerium oxide (MSN-Ce@SP/PEG). This nanoenzyme exhibited satisfactory catalase (CAT) activity, efficiently converting high levels of H2O2 within tumor cells into O2, effectively alleviating tumor hypoxia. Furthermore, MSN-Ce@SP/PEG nanoenzyme demonstrated high peroxidase (POD) activity, elevating reactive oxygen species (ROS) levels and attenuating AR in hepatocellular carcinoma (HCC) cells. The MSN-Ce@SP/PEG nanoenzyme exhibited satisfactory dual bioactivity in CAT and POD and was significantly enhanced under favorable photothermal conditions. Through the synergistic effects of these capabilities, the nanoenzyme disrupted the epithelial-mesenchymal transition (EMT) process in detached HCC cells, ultimately inhibiting the recurrence and metastasis potential of anoikis-resistant HCC cells. This study represents the first report of a novel nanoenzyme based on mesoporous silica/nano-cerium oxide for treating AR in HCC cells, thereby suppressing HCC recurrence and metastasis. The findings of this work offer a pioneering perspective for the development of innovative strategies to prevent the recurrence and metastasis of HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


