可见光促进喹喔啉-2(1H)-酮或香豆素与烷氧基丙烯酰氯的 C3-H 烷氧基羰基化反应。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们介绍了一种绿色高效的光氧化催化 C3-H 烷氧基羰基化反应,该反应是在环境条件下,将喹喔啉-2(1H)-酮或香豆素与容易获得的烷氧基羰基氯化物进行反应。通过原位生成的烷氧羰基自由基加成,制备出一系列喹喔啉-3-羰基和香豆素-3-羰基化合物。值得注意的是,该方法具有条件温和、操作简单、官能团耐受性好等特点。更重要的是,羧化产物可以很容易地衍生成其他重要化合物,这些化合物在开发具有医药活性的化合物方面具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
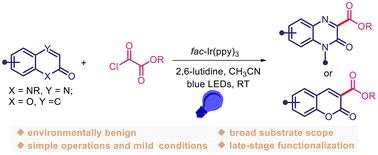
Visible light-promoted C3–H alkoxycarbonylation of quinoxalin-2(1H)-ones or coumarins with alkyloxalyl chlorides†
Herein, we describe a green and efficient photoredox catalytic C3–H alkoxycarbonylation between quinoxalin-2(1H)-ones or coumarins and readily available alkyloxalyl chlorides under ambient conditions. A series of quinoxaline-3-carbonyl and coumarin-3-carbonyl compounds are prepared through the radical addition of in situ-generated alkoxycarbonyl radicals. Notably, this protocol features mild conditions, operational simplicity, and excellent functional group tolerance. More importantly, the carboxylated products can be readily derivatized into other important compounds that would be of great potential for the exploitation of pharmaceutically active compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


