利用多模态成像质谱法揭示金黄色葡萄球菌骨髓炎的脂质动态变化
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
当金黄色葡萄球菌侵入骨骼微环境,导致骨髓脓肿,并形成细胞和生物大分子的空间结构时,就会发生骨髓炎。成像质谱法和显微镜是一种工具,可用于检测受金黄色葡萄球菌感染的小鼠股骨的脂质体,并揭示感染的代谢和信号转导后果。在这里,我们将近250种脂质与整个股骨的健康形态特征和感染相关形态特征进行了空间映射,建立了组织类型的成分概况。脓肿细胞和组织结构之间的醚脂和花生四烯醇脂发生了变化,表明它们在脓肿形成和炎症信号传导中的作用。甾醇、甘油三酯、双(单酰甘油)磷酸盐和神经节苷脂在整个脓肿中呈环状分布,这表明在传统显微镜下无法分辨的细胞群中存在脂质代谢失调的假说。这些数据让人们深入了解了脓肿纤维化边界细胞的信号功能和新陈代谢,这很可能是富含脂质的巨噬细胞的特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
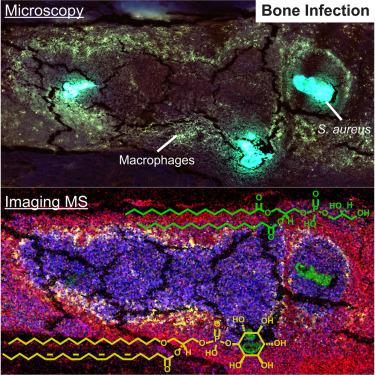
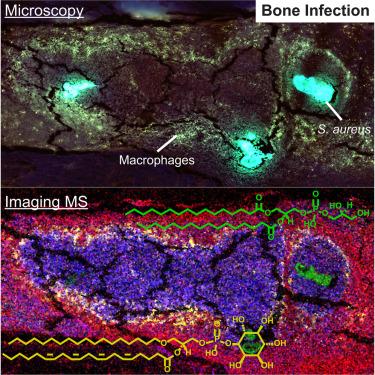
Uncovering lipid dynamics in Staphylococcus aureus osteomyelitis using multimodal imaging mass spectrometry
Osteomyelitis occurs when Staphylococcus aureus invades the bone microenvironment, resulting in a bone marrow abscess with a spatially defined architecture of cells and biomolecules. Imaging mass spectrometry and microscopy are tools that can be employed to interrogate the lipidome of S. aureus-infected murine femurs and reveal metabolic and signaling consequences of infection. Here, nearly 250 lipids were spatially mapped to healthy and infection-associated morphological features throughout the femur, establishing composition profiles for tissue types. Ether lipids and arachidonoyl lipids were altered between cells and tissue structures in abscesses, suggesting their roles in abscess formation and inflammatory signaling. Sterols, triglycerides, bis(monoacylglycero)phosphates, and gangliosides possessed ring-like distributions throughout the abscess, suggesting a hypothesized dysregulation of lipid metabolism in a population of cells that cannot be discerned with traditional microscopy. These data provide insight into the signaling function and metabolism of cells in the fibrotic border of abscesses, likely characteristic of lipid-laden macrophages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


