霍利迪连接解析酶RuvC靶向生物膜eDNA,使植物对维管束病原体产生抗性
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
生物膜生活方式对于细菌病原体在动植物中定植并保护自身免受宿主免疫和抗菌化学物质的侵害至关重要。植物细菌生物膜的形成和调控机制尚不清楚。在这里,我们发现 Ralstonia solanacearum 对紫外线 C 的抗性蛋白(RuvC)在生物膜中含量很高,并通过控制番茄木质部的系统运动来正向调节致病性。RuvC 蛋白在生物膜发育后期积累,并特异性地靶向霍利迪接合点(HJ)样结构,破坏生物膜胞外 DNA(eDNA)晶格,从而促进生物膜的扩散。重组 RuvC 蛋白可解决细胞外 HJ 问题,防止细菌形成生物膜。在番茄或水稻中异源表达带有植物分泌信号的 R. solanacearum 或 Xanthomonas oryzae pv. oryzae RuvC,可分别获得对细菌枯萎病或细菌性疫病的抗性。植物叶绿体定位的 HJ 分解酶单核叶绿体 1(MOC1)与细菌 RuvC 结构相似,对细菌生物膜的形成有很强的抑制作用。将 SlMOC1 重新定位到番茄根的细胞外质可增强对细菌枯萎病的抵抗力。我们的新发现揭示了 R. solanacearum 的关键致病机制,为提高植物对细菌性维管束病害的抗性提供了一种有效的生物技术策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Holliday junction resolvase RuvC targets biofilm eDNA and confers plant resistance to vascular pathogens
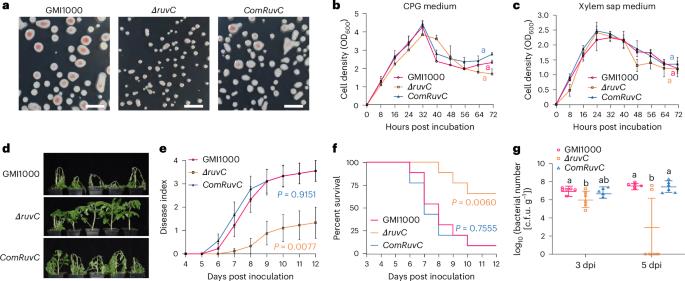
A biofilm lifestyle is critical for bacterial pathogens to colonize and protect themselves from host immunity and antimicrobial chemicals in plants and animals. The formation and regulation mechanisms of phytobacterial biofilm are still obscure. Here we found that the protein Ralstonia solanacearum resistance to ultraviolet C (RuvC) is highly abundant in biofilm and positively regulates pathogenicity by controlling systemic movement in tomato xylem. RuvC protein accumulates at the later stage of biofilm development and specifically targets Holliday junction (HJ)-like structures to disrupt the biofilm extracellular DNA (eDNA) lattice, thus facilitating biofilm dispersal. Recombinant RuvC protein can resolve extracellular HJ to prevent bacterial biofilm formation. Heterologous expression of R. solanacearum or Xanthomonas oryzae pv. oryzae RuvC with plant secretion signal in tomato or rice confers resistance to bacterial wilt or bacterial blight disease, respectively. Plant chloroplast-localized HJ resolvase monokaryotic chloroplast 1 (MOC1), which shares structural similarity with bacterial RuvC, shows a strong inhibitory effect on bacterial biofilm formation. Relocalization of SlMOC1 to apoplast in tomato roots leads to increased resistance to bacterial wilt. Our novel finding reveals a critical pathogenesis mechanism of R. solanacearum and provides an efficient biotechnology strategy to improve plant resistance to bacterial vascular disease. The bacterial pathogen Ralstonia solanacearum secretes endonuclease RuvC, which degrades mature biofilm by targeting the lattice formed by cruciform extracellular DNA. This helps bacterial dispersal, pathogen spread in plant xylem and virulence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


