以可逆共价方式靶向硫醇的新型亲电剂
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
具有可逆和共价相互作用优势的可逆共价亲电体在化学生物学和药物化学领域备受关注。在此,我们报告了两种由酰胺和酯活化的缺电子烯烃--酰胺取代的丙烯酰胺和甲酯取代的丙烯酰胺--以可逆共价方式靶向硫醇。本文章由计算机程序翻译,如有差异,请以英文原文为准。

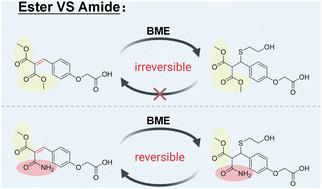
New electrophiles targeting thiols in a reversible covalent manner†
Reversible covalent electrophiles with the advantages of both reversible and covalent interactions receive much attention in the fields of chemical biology and medicinal chemistry. Here, we report two electron-deficient olefins activated by amide and ester, amide-substituted acrylamide and methyl ester-substituted acrylamide, targeting thiols in a reversible covalent manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


