从内酯原料合成聚硫醚的通用温和方法
IF 5.2
Q1 POLYMER SCIENCE
引用次数: 0
摘要
在要求可降解或可回收的应用领域,聚硫醚正引起越来越多的关注。然而,合成这些聚合物的通用方法却寥寥无几。本报告介绍了一种利用容易获得的内酯原料合成聚硫醚的快速而通用的方法。该方法分两步进行,首先将内酯亚硫酰化为亚硫酰内酯,然后将亚硫酰内酯开环聚合为聚硫酯。与以往依赖刺激性试剂完成这种转化的方法不同,我们证明了温和的硫代乙酸四丁基铵是一种合格的聚合引发剂。这种方法具有广泛的适用性,成功聚合了一个未受约束的 17 元大环和一个 N-取代环硫代氨基甲酸酯就证明了这一点。此外,这种方法的通用性使得它能够合成具有高度可调特性的聚硫酯,合成出一组玻璃化转变温度超过 180 °C 的聚合物就证明了这一点。最后,聚硫醚可有效地解聚成相应的硫内酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
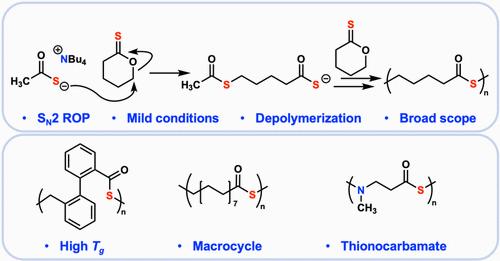
General and Mild Method for the Synthesis of Polythioesters from Lactone Feedstocks
Polythioesters are attracting increasing interest in applications requiring degradability or recyclability. However, few general methods exist for the synthesis of these polymers. This report presents a fast and versatile method for synthesizing polythioesters from readily available lactone feedstocks. The two-step process begins with the thionation of lactones to thionolactones, followed by the ring-opening polymerization of the thionolactones to polythioesters. Unlike previous methods that rely on harsh reagents to accomplish this transformation, we demonstrate that the mild tetrabutylammonium thioacetate is a competent initiator for polymerization. This method exhibits broad applicability, as demonstrated by the successful polymerizations of an unstrained 17-membered macrocycle and an N-substituted cyclic thionocarbamate. Furthermore, the generality of this scheme enables the synthesis of polythioesters with highly tunable properties, as demonstrated here by the synthesis of a set of polymers with glass transition temperatures spanning 180 °C. Finally, the polythioesters are efficiently depolymerized into the corresponding thiolactones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


