白色念珠菌药物靶标中的大多数唑类抗性突变都会产生交叉抗性,而不会带来内在的健康代价
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
唑类抗真菌药是治疗真菌感染的主要药物。药物靶点 Erg11(Cyp51)中的氨基酸取代是病原酵母菌常见的抗药性机制。然而,究竟有多少突变以及哪些突变会产生耐药性在很大程度上还是未知数。在这里,我们测量了白念珠菌 Erg11 近 4000 个氨基酸变体对六种临床唑类药物敏感性的影响。这是通过对在酿酒酵母中表达的 CaErg11 进行深度突变扫描实现的。我们发现,很大一部分突变会导致抗性(33%),大多数抗性突变会产生交叉抗性(88%),只有少数抗性突变显示出显著的适应性代价(9%)。我们的研究结果表明,唑类药物的抗药性可通过大量突变产生,这很可能会导致唑类药物的泛抗药性,而几乎不会影响进化。这一资源将有助于为临床治疗选择提供依据,并为新药开发提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。

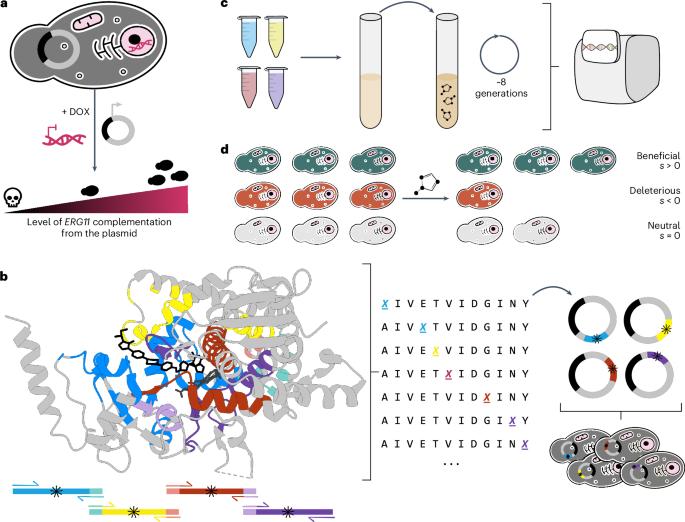
Most azole resistance mutations in the Candida albicans drug target confer cross-resistance without intrinsic fitness cost
Azole antifungals are the main drugs used to treat fungal infections. Amino acid substitutions in the drug target Erg11 (Cyp51) are a common resistance mechanism in pathogenic yeasts. How many and which mutations confer resistance is, however, largely unknown. Here we measure the impact of nearly 4,000 amino acid variants of Candida albicans Erg11 on the susceptibility to six clinical azoles. This was achieved by deep mutational scanning of CaErg11 expressed in Saccharomyces cerevisiae. We find that a large fraction of mutations lead to resistance (33%), most resistance mutations confer cross-resistance (88%) and only a handful of resistance mutations show a significant fitness cost (9%). Our results reveal that resistance to azoles can arise through a large set of mutations and this will probably lead to azole pan-resistance, with little evolutionary compromise. This resource will help inform treatment choices in clinical settings and guide the development of new drugs. Deep mutational scanning of the azole antifungals drug target Erg11 provides an extensive catalogue of resistance mutations and reveals that resistance to azoles can arise through a large set of mutations that will probably lead to azole pan-resistance without a fitness cost.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


