可溶性蛋白质选择性结合β-胡萝卜素的结构基础
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
β-胡萝卜素(BCR)是最丰富的类胡萝卜素,是一种着色剂、抗氧化剂和维生素 A。这种碳氢化合物的疏水性极强,需要特殊的机制才能在水介质中分布,包括水溶性胡萝卜素蛋白。然而,所有已知的类胡萝卜素蛋白都偏爱含氧类胡萝卜素,与 BCR 的结合效率很低。在这里,我们展示了雄性蝗虫群居时产生的 BCR 结合蛋白(BBP)与其天然配体 BCR 复合物的晶体结构。BBP 形成了一个反平行管状同源二聚体,其中有 α/β 缠绕折叠的单体,每个单体都形成了一个 47 Å 长的疏水同轴隧道,该隧道向外开放,被一个 s-cisC6-C7 全反式 BCR 分子占据。在缺乏 BCR 的情况下,BBP 可接受一系列黄绿素,但效率会因氧原子的位置和数量而降低,但会排斥番茄红素。该结构捕捉到了色素与 Takeout 1 蛋白的复合物,激发了 BBP 作为 BCR 增溶剂的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
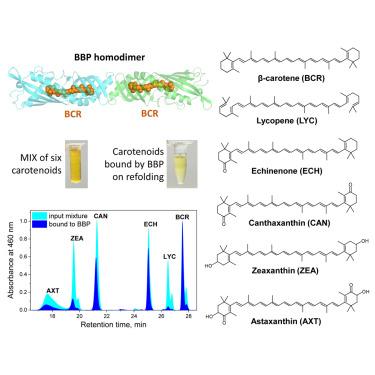
Structural basis of selective beta-carotene binding by a soluble protein
β-carotene (BCR) is the most abundant carotenoid, a colorant, antioxidant, and provitamin A. The extreme hydrophobicity of this hydrocarbon requires special mechanisms for distribution in aqueous media, including water-soluble carotenoproteins. However, all known carotenoproteins prefer oxygenated carotenoids and bind BCR inefficiently. Here, we present the crystal structure of the BCR-binding protein (BBP) from gregarious male locusts, which is responsible for their vivid yellow body coloration, in complex with its natural ligand, BCR. BBP forms an antiparallel tubular homodimer with α/β-wrap folded monomers, each forming a hydrophobic 47 Å long, coaxial tunnel that opens outward and is occupied by one s-cisC6-C7, all-trans BCR molecule. In the BCR absence, BBP accepts a range of xanthophylls, with reduced efficiency depending on the position and number of oxygen atoms, but rejects lycopene. The structure captures a pigment complex with a Takeout 1 protein and inspires potential applications of BBP as a BCR solubilizer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


