产气荚膜梭菌肠毒素与其人类受体 Claudin-4 结合的冷冻电子显微镜结构
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
产气荚膜梭状芽孢杆菌肠毒素(CpE)可导致常见的致命性胃肠道疾病。CpE 会与小肠上皮细胞顶端表面上名为 Claudins 的受体结合。Claudins通常调节细胞外转运,但被CpE劫持后,Claudin/CpE复合物形成。Claudin/CpE复合物是低聚β桶状孔的构件,可穿透质膜并诱导肠道细胞毒性。在这里,我们利用低温电子显微镜展示了 CpE 与人类原生克劳丁受体克劳丁-4 复合物的结构。这些结构揭示了克劳丁/CpE 复合物的结构、用于结合的残基、CpE 相对于膜的取向以及 CpE 诱导的克劳丁-4 变化。此外,结构和建模暗示了在致病过程中从 claudin/CpE 复合物到细胞毒性 β 桶孔的生物物理过程。总之,这项研究提出了一个克劳丁/CpE组装模型,并提供了阻碍其形成以治疗CpE疾病的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
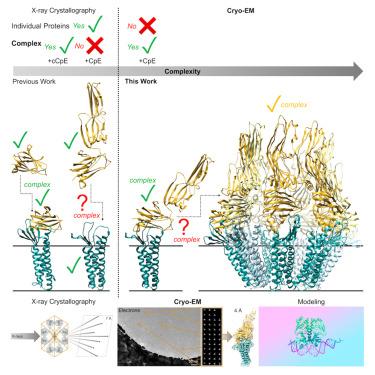
Cryo-EM structures of Clostridium perfringens enterotoxin bound to its human receptor, claudin-4
Clostridium perfringens enterotoxin (CpE) causes prevalent and deadly gastrointestinal disorders. CpE binds to receptors called claudins on the apical surfaces of small intestinal epithelium. Claudins normally regulate paracellular transport but are hijacked from doing so by CpE and are instead led to form claudin/CpE complexes. Claudin/CpE complexes are the building blocks of oligomeric β-barrel pores that penetrate the plasma membrane and induce gut cytotoxicity. Here, we present the structures of CpE in complex with its native claudin receptor in humans, claudin-4, using cryogenic electron microscopy. The structures reveal the architecture of the claudin/CpE complex, the residues used in binding, the orientation of CpE relative to the membrane, and CpE-induced changes to claudin-4. Further, structures and modeling allude to the biophysical procession from claudin/CpE complexes to cytotoxic β-barrel pores during pathogenesis. In full, this work proposes a model of claudin/CpE assembly and provides strategies to obstruct its formation to treat CpE diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


