Vipp1 膜结合的结构基础:从松散的外套和地毯到环状和杆状组件
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
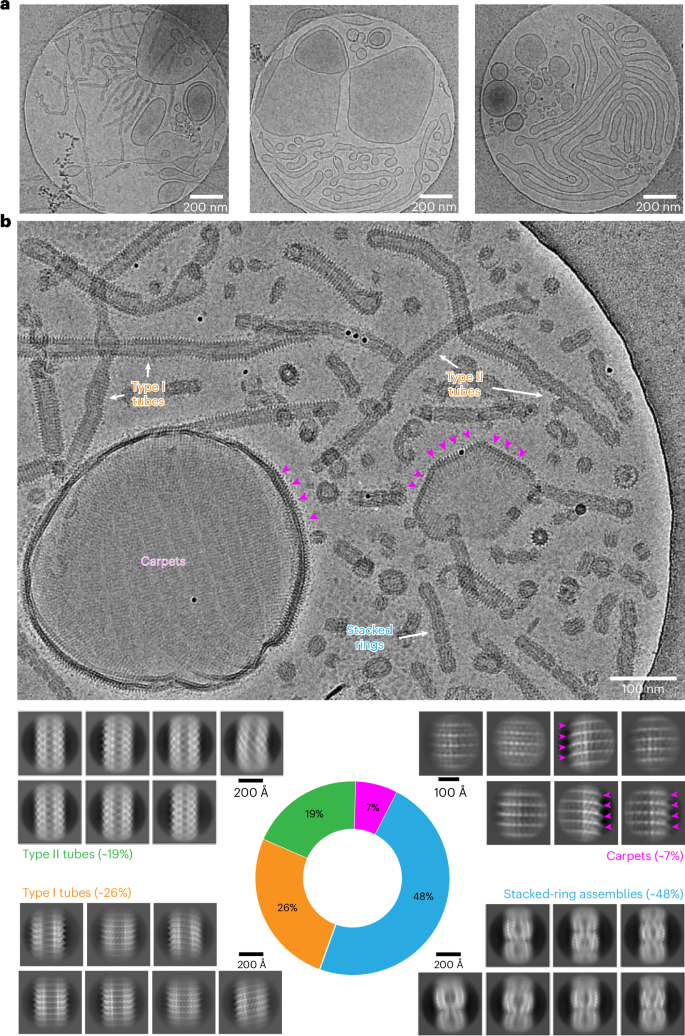
质体中的囊泡诱导蛋白 1(Vipp1)对类囊体膜的生物发生和维持至关重要。虽然 Vipp1 最近被确定为运输 III 超家族所需的内质体分选复合物的成员,但 Vipp1 如何重塑膜仍是未知数。在此,我们展示了 Synechocystis Vipp1 与膜相互作用的冷冻电镜结构:7 个 5-7 Å 分辨率的吞噬膜的螺旋和叠环组装结构,以及 3 个使用子图平均法、约 20 Å 分辨率的覆盖脂质囊泡的地毯结构。通过分析 N 端截短的 Vipp1 的十个结构,我们发现螺旋 α0 对于膜管化至关重要,并形成了 Vipp1 的膜锚定结构域。最后,我们利用构象受限的 Vipp1 突变体,降低了 Vipp1 的结构可塑性,并以 3.0 Å 的分辨率测定了 Vipp1 的两个结构,解析了螺旋 α0 的膜锚定和亚基间接触的分子细节。我们的数据揭示了从地毯到环和杆的膜曲率依赖性结构转换,其中一些结构转换能够诱导和/或稳定引发膜融合的高局部膜曲率。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Structural basis for Vipp1 membrane binding: from loose coats and carpets to ring and rod assemblies
Vesicle-inducing protein in plastids 1 (Vipp1) is critical for thylakoid membrane biogenesis and maintenance. Although Vipp1 has recently been identified as a member of the endosomal sorting complexes required for transport III superfamily, it is still unknown how Vipp1 remodels membranes. Here, we present cryo-electron microscopy structures of Synechocystis Vipp1 interacting with membranes: seven structures of helical and stacked-ring assemblies at 5–7-Å resolution engulfing membranes and three carpet structures covering lipid vesicles at ~20-Å resolution using subtomogram averaging. By analyzing ten structures of N-terminally truncated Vipp1, we show that helix α0 is essential for membrane tubulation and forms the membrane-anchoring domain of Vipp1. Lastly, using a conformation-restrained Vipp1 mutant, we reduced the structural plasticity of Vipp1 and determined two structures of Vipp1 at 3.0-Å resolution, resolving the molecular details of membrane-anchoring and intersubunit contacts of helix α0. Our data reveal membrane curvature-dependent structural transitions from carpets to rings and rods, some of which are capable of inducing and/or stabilizing high local membrane curvature triggering membrane fusion. The authors present structures of endosomal sorting complexes required for transport III family member vesicle-inducing protein in plastids 1, ranging from helical assemblies and stacked rings to flat carpets, providing insights into transitions dependent on membrane tubulation and curvature needed for forming different architectures involved in membrane remodeling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


