利用钴镍双金属改性 Gd2O3&Co3O4 纳米复合材料实现高效、选择性光热催化 CO2 还原成 CH4
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
随着大气中二氧化碳含量的增加,如何将二氧化碳转化为燃料已成为研究热点。光热催化是通过萨巴蒂尔反应获得 CH4 的一种颇具前景的方法。在这种情况下,迫切需要设计高效的催化剂来提高水性 CO2 到 CH4 的转化率和选择性。本研究通过煅烧和化学沉积方法制备了 CoNi/(Gd2O3&Co3O4)纳米复合材料。与 Gd2O3 和 Co3O4 相比,80%-CoNi/(Gd2O3&Co3O4)具有更高的光热催化 CH4 演化率(4.19 mmol g-1 h-1)和对 CH4 的高选择性(98.6%)。此外,该样品还具有出色的持久性和循环催化稳定性。结合系统分析发现,80%-CoNi/(Gd2O3&Co3O4)催化剂的优异催化性能主要归因于两个方面:(i) P-N 和肖特基结形成的双异质结构使催化剂具有很高的电荷分离效率;(ii) 催化剂具有优异的 CO2 吸附能力和氧化还原能力,大大提高了催化活性。这项研究对二氧化碳的资源化利用和催化剂的可持续利用具有重大意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
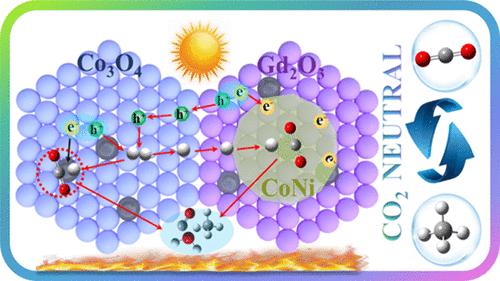
Highly Efficient and Selective Photothermal Catalytic CO2 Reduction to CH4 Using the CoNi Bimetallic-Modified Gd2O3&Co3O4 Nanocomposite
With the increase of CO2 in the atmosphere, how to convert CO2 into fuel has become a research hotspot. Photothermal catalysis is a quite promising method to obtain CH4 via the Sabatier reaction. In this context, it is urgent to design efficient catalysts to improve the conversion and selectivity of aqueous CO2 to CH4. In this work, the CoNi/(Gd2O3&Co3O4) nanocomposite was fabricated via calcination and chemical deposition methods. Compared with Gd2O3 and Co3O4, 80%-CoNi/(Gd2O3&Co3O4) shows an enhancement of photothermal catalytic CH4 evolution rate (4.19 mmol g–1 h–1) and high selectivity for CH4 (98.6%). In addition, this sample also possesses outstanding durable and cyclic catalytic stability. Combined with systematic analysis, it was found that the excellent catalytic performance of 80%-CoNi/(Gd2O3&Co3O4) can be attributed to two main reasons: (i) the dual-heterostructure formation of the P–N and Schottky junction endows the catalyst with high charge separation efficiency and (ii) the catalyst presents preeminent CO2 adsorption and redox capacities, which greatly improves its catalytic activity. This study has significant advantages for the resource utilization of CO2 and the sustainable utilization of catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


