氢化铜(I)催化甲酸脱氢的机理研究
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
众所周知,氢化物铜(I)络合物通常会与二氧化碳反应生成相应的甲酸铜。然而,最近观察到一些多核氢化物铜具有相反的反应性,可以催化甲酸的脱氢反应。在此,我们报告了使用多核 PNNP 氢化物铜配合物作为该反应的活性(前)催化剂的情况。机理研究深入揭示了催化剂静止状态和速率决定步骤,并确定了一种非循环物种,该物种是导致该反应中出现意外底物抑制的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
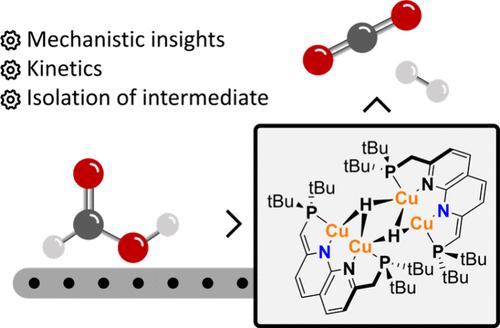
Mechanistic Investigation into Copper(I) Hydride Catalyzed Formic Acid Dehydrogenation
Copper(I) hydride complexes are typically known to react with CO2 to form their corresponding copper formate counterparts. However, recently it has been observed that some multinuclear copper hydrides can feature the opposite reactivity and catalyze the dehydrogenation of formic acid. Here we report the use of a multinuclear PNNP copper hydride complex as an active (pre)catalyst for this reaction. Mechanistic investigations provide insights into the catalyst resting state and the rate-determining step and identify an off-cycle species that is responsible for the unexpected substrate inhibition in this reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


