优化Irf8 +32-kb增强子可破坏树突状细胞系的分离
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
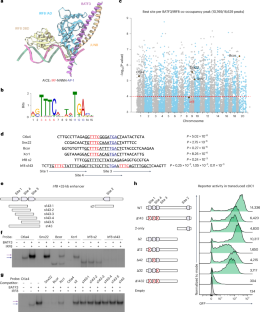
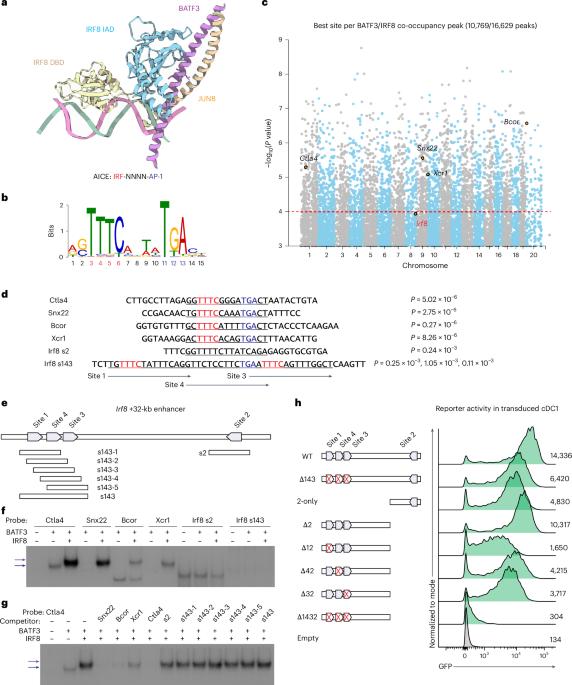
细胞系决定转录因子的自动激活介导了双稳态表达,产生了对复杂身体计划至关重要的不同细胞表型。经典的 1 型树突状细胞(cDC1)和 2 型树突状细胞(cDC2)亚群具有非冗余的功能,可抵御不同的免疫挑战。干扰素调节因子8(IRF8)是决定cDC1品系的转录因子,它在cDC1祖细胞中自动激活以建立cDC1特性,但在cDC2分化过程中其表达却因未知机制而下调。本研究揭示了负责IRF8自动激活的Irf8 +32-kb增强子与低亲和力IRF8结合位点的天然次优化。在Irf8 +32-kb增强子中引入多个高亲和性IRF8位点会产生功能增益效应,导致特定的cDC2祖细胞中的IRF8自动激活发生错误,使它们转向cDC1和具有混系表型的新型杂交DC亚群。此外,这还会导致功能缺失效应,减少 cDC1 中 Irf8 的表达。这些发育改变严重损害了 cDC1 依赖性免疫和 cDC2 依赖性免疫。总之,我们的发现强调了增强子次优化在正常免疫功能所需的 cDCs 发育分离中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimization of the Irf8 +32-kb enhancer disrupts dendritic cell lineage segregation
Autoactivation of lineage-determining transcription factors mediates bistable expression, generating distinct cell phenotypes essential for complex body plans. Classical type 1 dendritic cell (cDC1) and type 2 dendritic cell (cDC2) subsets provide nonredundant functions for defense against distinct immune challenges. Interferon regulatory factor 8 (IRF8), the cDC1 lineage-determining transcription factor, undergoes autoactivation in cDC1 progenitors to establish cDC1 identity, yet its expression is downregulated during cDC2 differentiation by an unknown mechanism. This study reveals that the Irf8 +32-kb enhancer, responsible for IRF8 autoactivation, is naturally suboptimized with low-affinity IRF8 binding sites. Introducing multiple high-affinity IRF8 sites into the Irf8 +32-kb enhancer causes a gain-of-function effect, leading to erroneous IRF8 autoactivation in specified cDC2 progenitors, redirecting them toward cDC1 and a novel hybrid DC subset with mixed-lineage phenotypes. Further, this also causes a loss-of-function effect, reducing Irf8 expression in cDC1s. These developmental alterations critically impair both cDC1-dependent and cDC2-dependent arms of immunity. Collectively, our findings underscore the significance of enhancer suboptimization in the developmental segregation of cDCs required for normal immune function. Some enhancers can limit their activities to specific spatial–temporal domains by enhancer suboptimization. Ou et al. find that classical dendritic cell (cDC) development depends on Irf8 suboptimization, which prevents unwanted IRF8 autoactivation in developing cDC2s while maximizing IRF8 expression in developed cDC1s.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


