用于耐多药细菌感染特异性监测和治疗的多功能自组装近红外 SERS 纳米探针
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
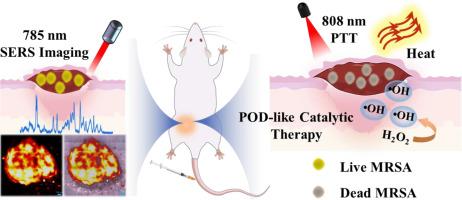
多重耐药菌(MDRB)的兴起使细菌感染成为最大的健康威胁之一,导致大量抗生素失效。因此需要对感染部位的细菌疾病治疗效果进行实时监测。在此,我们报告了一种多功能拉曼标记 3,3'-二乙基噻三碳菁碘化物(DTTC)-共轭星形金-MoS2@透明质酸(AMD@HA)纳米复合材料,作为一种表面增强拉曼散射(SERS)纳米探针,用于快速识别细菌并进行原位根除。混合金属纳米结构产生的局部表面等离子体共振(LSPR)使AMD@HA对近红外激光具有很高的响应性,从而使其表现出光热效应(PTT)、更高的SERS活性以及类似过氧化物酶的催化反应以释放活性氧。尾静脉注射 AMD@HA 纳米探针具有创伤性,而用于细菌鉴定的 SERS 成像则具有非创伤性和灵敏性,因此是一种高效的残留细菌监测方法。耐甲氧西林金黄色葡萄球菌(MRSA)的检测限低至 102 CFU-mL-1,而基底可在 120 秒内获得 1,600 (40 × 40) 像素的拉曼图像。在 MRSA 引起的伤口感染和皮肤脓肿的小鼠模型中,AMD@HA 介导的 PTT 和催化治疗相结合,在通过快速杀菌促进伤口愈合方面显示出协同效应。这种 SERS 引导的治疗方法毒性小,不会造成严重的附带损害,为治疗由 MDRB 引起的疾病提供了一种极具前景的干预方法。意义说明:这项研究引入了一种 SERS 纳米探针 AMD@HA,用于快速识别和根除多重耐药菌(MDRB)这一严重的健康威胁。该纳米探针利用局部表面等离子体共振进行光热治疗和增强拉曼信号,提供了一种灵敏、无创的诊断工具。我们的方法对 MRSA 的检测限很低,而且在小鼠模型中具有协同治疗效果,因此有望以最小的毒性治疗 MDRB 驱动的感染,推动抗菌策略领域的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Versatile self-assembled near-infrared SERS nanoprobes for multidrug-resistant bacterial infection-specific surveillance and therapy
The rise of multidrug-resistant bacteria (MDRB) has made bacterial infection one of the biggest health threats, causing numerous antibiotics to fail. Real-time monitoring of bacterial disease treatment efficacy at the infection site is required. Herein, we report a versatile Raman tag 3,3′-diethylthiatricarbocyanine iodide (DTTC)-conjugated star-shaped Au-MoS2@hyaluronic acid (AMD@HA) nanocomposite as a surface-enhanced Raman scattering (SERS) nanoprobe for quick bacterial identification and in-situ eradication. Localized surface plasmon resonance (LSPR) from the hybrid metallic nanostructure makes AMD@HA highly responsive to the near-infrared laser, enabling it to demonstrate a photothermal (PTT) effect, increased SERS activity, and peroxidase-like catalytic reaction to release reactive oxygen species. The tail vein injection of AMD@HA nanoprobes is invasive, however SERS imaging for bacterial identification is non-invasive and sensitive, making it an efficient residual bacteria monitoring method. The detection limit for methicillin-resistant Staphylococcus aureus (MRSA) is as low as 102 CFU·mL-1, and the substrates allow for taking 120 s to acquire a Raman image of 1600 (40 × 40) pixels. In mouse models of MRSA-induced wound infection and skin abscess, the combination of AMD@HA-mediated PTT and catalytic therapy demonstrates a synergistic effect in promoting wound healing through rapid sterilization. This SERS-guided therapeutic approach exhibits little toxicity and does not cause considerable collateral damage, offering a highly promising intervention for treating diseases caused by MDRB.
Statement of significance
This research introduces a SERS nanoprobe, AMD@HA, for the rapid identification and eradication of multidrug-resistant bacteria (MDRB), a critical health threat. The nanoprobe leverages localized surface plasmon resonance for photothermal therapy and enhanced Raman signals, offering a sensitive, non-invasive diagnostic tool. With a low detection limit for MRSA and a synergistic therapeutic effect in mouse models, our approach holds significant promise for treating MDRB-driven infections with minimal toxicity, advancing the field of antimicrobial strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


