开发用于持续眼部给药的葛根素负载聚(乳酸)微球:体外/体内评估。
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-05
DOI:10.1016/j.ejpb.2024.114524
引用次数: 0
摘要
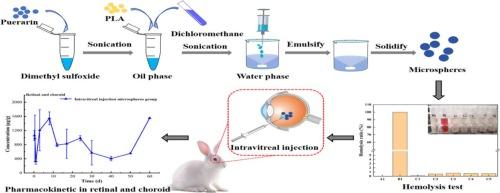
糖尿病视网膜病变是糖尿病的眼部并发症,是导致成人失明的重要原因。葛根素被认为具有治疗糖尿病视网膜病变的临床应用潜力。在这项研究中,我们设计了一种适合眼部给药的新型葛根素负载型聚乳酸缓释微球,并对其体外和体内特性进行了评估。通过盒-贝肯响应面设计优化了葛根素载药微球的制备。微球的包封效率和载药量分别为 35.71% 和 3.85%。微球具有良好的分散性和较高的安全性,适用于眼部给药。体外释放表明,微球具有良好的持续释放效果,其释放行为符合零阶动力学特征。眼组织分布结果显示,微球组在视网膜和脉络膜中的Cmax和AUC0-∞明显高于溶液组和静脉注射组。该研究表明,微球玻璃体内注射能显著延长葛根素在眼组织中的半衰期,实现药物的持续释放。因此,微球玻璃体内注射对糖尿病视网膜病变的治疗具有积极意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development of puerarin-loaded poly(lactic acid) microspheres for sustained ocular delivery: In vitro/vivo evaluation
Diabetic retinopathy, an ocular complication of diabetes, is an important cause of blindness in adults. Puerarin is considered to have promising potential for clinical use in treating diabetic retinopathy. In this study, we designed a novel puerarin-loaded poly(lactic acid) sustained-release microspheres suitable for ocular administration, and we assessed its in vitro and in vivo properties. The preparation of puerarin-loaded microspheres was optimized by Box-Behnken response surface design. The encapsulation efficiency and drug loading of microspheres were 35.71% and 3.85%, respectively. The microspheres exhibited good dispersion and high safety, making it suitable for ocular drug delivery. In vitro release demonstrated that microspheres had a well-sustained release effectiveness, and its release behavior complied with the zero-order kinetic characteristics. The results of ocular tissue distribution revealed that the Cmax and AUC0-∞ of the microspheres group in the retina and choroid were considerably higher than those of the solution group and the intravenous injection group. This research revealed that intravitreal injection of microspheres can significantly prolong the half-life of puerarin in eye tissues and achieve sustained drug release. Therefore, intravitreal injection of microspheres has positive implications for the treatment of diabetic retinopathy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


