质谱法反映了铜淀粉样蛋白 β 化学的关键方面。
IF 3.6
3区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
质谱法是一种研究蛋白质复合物的强大方法;然而,生化反应通常超出了质谱法的研究范围。在这里,我们研究了[铜(II) - 淀粉样蛋白 β]复合物的气相氧化还原化学反应,结果表明,这种化学反应的序列依赖性反映了肽的不同变体的已知体外行为的关键方面。本文章由计算机程序翻译,如有差异,请以英文原文为准。
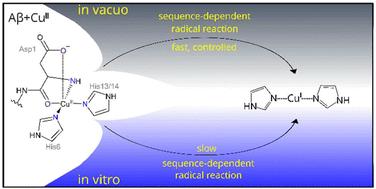
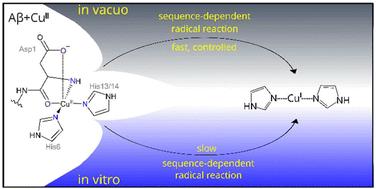
Mass spectrometry reflects key aspects of copper-amyloid β chemistry†
Mass spectrometry is a powerful method to study protein complexes; however, biochemical reactions are typically beyond the scope of MS studies. Here, we have studied the gas-phase redox chemistry of the [copper(II) – amyloid β] complex and show that the sequence-dependence of this chemistry reflects key aspects of the known in vitro behaviour of different variants of the peptide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analyst
化学-分析化学
CiteScore
7.80
自引率
4.80%
发文量
636
审稿时长
1.9 months
期刊介绍:
"Analyst" journal is the home of premier fundamental discoveries, inventions and applications in the analytical and bioanalytical sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


