毛细管电泳法,用于定量测定胶束形式的疏水性透明质酸。
IF 2.6
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
基于疏水性透明质酸(HA)的胶束常用于靶向给药系统。毛细管区带电泳(CZE)被用于定量测定疏水化透明质酸和原生透明质酸。所开发的通用方法适用于定量分析透明质酸的两亲衍生物(油酰透明质酸和与萘二甲酰亚胺荧光团共轭的透明质酸)以及不同分子量(15、150 和 800 kDa)的原生 HA。此外,还提出了基于油酰基透明质酸的胶束形式同时定量药物物质和油酰基透明质酸的方法。CE 技术被用于分析两种带相反电荷离子形式的难溶性药物(洛哌丁胺和利福布丁)的油酰透明质酸胶束形式。研究中的实例表明,透明质酸的分析灵敏度(LOD)范围为 11 至 40 μg/mL,药物的分析灵敏度(LOD)范围为 0.4 至 0.6 μg/mL。该研究还展示了基于油酰基透明质酸胶束形式的利福布汀和洛哌丁胺的精确定量测定,无需样品制备。因此,所提出的方法可用于定量原生 HA 或其两亲衍生物,并同时测定不同性质的药物物质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
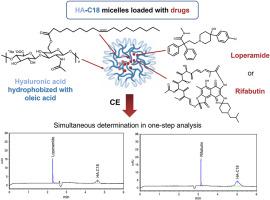
The method of capillary electrophoresis for quantitative determination of hydrophobized hyaluronic acid in its micellar forms
Micelles based on hydrophobized hyaluronic acid (HA) are frequently used in targeted drug delivery systems. Capillary zone electrophoresis (CZE) was utilized for the quantitative determination of hydrophobized and native HA. A universal methodology was developed, suitable for the quantitative analysis of amphiphilic derivatives of hyaluronan (oleyl hyaluronan and hyaluronan conjugate with naphthalimide fluorophore) and native HA with varying molecular weights (15, 150, and 800 kDa). Furthermore, methodologies were proposed for the simultaneous quantification of a drug substance and oleyl hyaluronan in micellar forms based on the latter. The CE technique was applied for analyzing oleyl-hyaluronan-based micellar forms of two poorly soluble drug substances with oppositely charged ionic forms (loperamide and rifabutin). The examples contained in the study demonstrate a range of analytical sensitivity (LOD) for hyaluronan from 11 to 40 μg/mL and for the drug substance from 0.4 to 0.6 μg/mL. The study also showcases the accurate quantitative determination of rifabutin and loperamide in oleyl-hyaluronan-based micellar forms without the need for sample preparation. Thus, the proposed methodologies can be used to quantify native HA or its amphiphilic derivatives and simultaneously determine drug substances of various nature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


