近红外远程触发生物酶激活,控制口服微生物的肠道定植。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
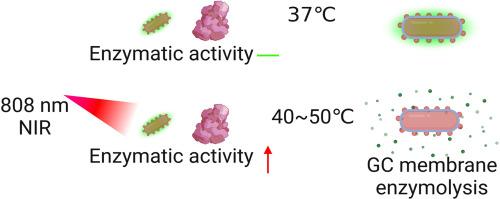
口服生物疗法前景广阔,但其缺乏可控性和靶向性,尤其是对肠道细菌生物疗法而言,是一项重大挑战。为此,我们开发了一种纳米封装方法,它能对生物体内酶活性的释放做出反应,并在原位激活酶,从而实现肠道微生物的可控定植。这种纳米涂层由两层结构组成:内层是具有光热和粘合特性的聚多巴胺,外层是明胶-羧甲基纤维素钠,在与多巴胺发生光热作用后,纤维素酶会在肠道中水解。我们成功实现了多种微生物的可控定植。此外,在糖尿病模型中,这种方法对调节胰高血糖素样肽-1(GLP-1)的产生、β细胞生理机能和促进胰岛素分泌产生了深远影响。这种纳米涂层是通过原位激活纤维素酶实现的,无需进行基因或靶向分子改造,是微生物疗法的一种新模式和替代策略。它不仅实现了益生菌的精确和可控定植,还展示了在口腔生物疗法领域更广泛应用的巨大潜力。意义说明:我们开发了一种纳米封装方法,它能触发酶活性,从而控制肠道内多种微生物的释放和粘附。纳米涂层由两层组成:内层是具有光热和粘附特性的聚多巴胺,外层是明胶-羧甲基纤维素钠聚合物,可在肠道中被纤维素酶水解。此外,这种方法还可以制备各种微生物涂层。这种方法在调节 GLP-1 的产生、胰腺 β 细胞的生理功能以及促进糖尿病模型的胰岛素分泌方面大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Near-infrared remote triggering of bio-enzyme activation to control intestinal colonization by orally administered microorganisms
Oral biotherapeutics hold significant promise, but their lack of controllability and targeting poses a major challenge, particularly for intestinal bacterial biotherapeutics. In response, we have developed a nanoencapsulation approach that responds to the release of enzyme activity in the organism and activates the enzyme in situ, allowing for controlled colonization of microbes in the gut. The nano-coating comprises a two-layer structure: an inner layer of polydopamine with photothermal and adhesive properties, and an outer layer of gelatin–sodium carboxymethylcellulose, which is hydrolyzed by cellulases in the gut following photothermal interaction with dopamine. We have successfully achieved controlled colonization of a wide range of microorganisms. Furthermore, in a diabetes model, this approach has had a profound impact on regulating glucagon-like peptide-1 (GLP-1) production, β-cell physiology, and promoting insulin secretion. This nanocoating is achieved by in situ activation of cellulase without the need for genetic or targeted molecular modification, representing a new paradigm and alternative strategy for microbial therapy. It not only enables precise and controlled colonization of probiotics but also demonstrates great potential for broader application in the field of oral biotherapy.
Statement of significance
We have developed a nano-encapsulation method that triggers enzyme activity in response to enzymatic activity, resulting in the controlled release and adhesion of a wide range of microorganisms in the gut. The nano coating comprises two layers: an inner layer of polydopamine with photothermal and adhesion properties, and an outer layer of a gelatin-sodium carboxymethylcellulose polymer, which can be hydrolyzed by cellulases in the intestine. Additionally, this method allows for the preparation of various microbial coatings. This approach holds significant promise for regulating GLP-1 production, the physiological function of pancreatic β-cells, and promoting insulin secretion in diabetes models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


