三维水凝胶中的细胞机械刺激光学系统
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
我们介绍了一种利用单个激光产生的空化气泡来刺激嵌入三维水凝胶中的真皮成纤维细胞的细胞机械传导的方法。我们证明,嵌入无定形或纤维状水凝胶中的成纤维细胞在受到直径为 250 微米的单个激光产生的空化泡提供的脉冲机械刺激后,会产生 Ca2+ 信号。我们发现,与嵌入无定形聚乙烯醇聚合物(SLO-PVA)水凝胶中的细胞相比,嵌入纤维胶原水凝胶中的细胞发出信号的空间范围更大。此外,对于包埋在胶原蛋白中的成纤维细胞,我们发现与圆周轴相比,相对于径向轴极化的细胞的机械敏感性范围更大。相比之下,包埋在 SLO-PVA 中的成纤维细胞没有显示出取向依赖性机械敏感性。嵌入水凝胶并在无钙培养基中培养的成纤维细胞没有表现出空化诱导的机械传导;这说明钙信号转导是基于跨膜 Ca2+ 转运的。这项研究表明,单个激光产生的空化气泡可在三维水凝胶组织模型中提供局部非侵入性脉冲机械刺激,并同时使用光学显微镜成像。意义说明目前,对三维组织支架内细胞对机械刺激的敏感性进行非侵入式实时评估的方法非常有限。我们介绍了一种独创的方法,它利用标准激光扫描显微镜系统中的脉冲激光微束产生单个空化气泡,为三维纤维状和无定形水凝胶中的细胞提供脉冲机械刺激。利用这种技术,我们测量了嵌入无定形和纤维状水凝胶中的原代人类真皮成纤维细胞的细胞机械敏感性,从而为研究三维生物材料中的细胞机械传导提供了一种有用的方法。此外,我们的方法可在标准光学显微镜中实现,因此适合细胞机械传导研究人员广泛采用,并为高通量评估生物材料的细胞机械信号提供了可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
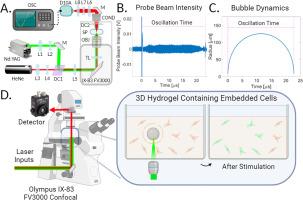
An optical system for cellular mechanostimulation in 3D hydrogels
We introduce a method utilizing single laser-generated cavitation bubbles to stimulate cellular mechanotransduction in dermal fibroblasts embedded within 3D hydrogels. We demonstrate that fibroblasts embedded in either amorphous or fibrillar hydrogels engage in Ca2+ signaling following exposure to an impulsive mechanical stimulus provided by a single 250 µm diameter laser-generated cavitation bubble. We find that the spatial extent of the cellular signaling is larger for cells embedded within a fibrous collagen hydrogel as compared to those embedded within an amorphous polyvinyl alcohol polymer (SLO-PVA) hydrogel. Additionally, for fibroblasts embedded in collagen, we find an increased range of cellular mechanosensitivity for cells that are polarized relative to the radial axis as compared to the circumferential axis. By contrast, fibroblasts embedded within SLO-PVA did not display orientation-dependent mechanosensitivity. Fibroblasts embedded in hydrogels and cultured in calcium-free media did not show cavitation-induced mechanotransduction; implicating calcium signaling based on transmembrane Ca2+ transport. This study demonstrates the utility of single laser-generated cavitation bubbles to provide local non-invasive impulsive mechanical stimuli within 3D hydrogel tissue models with concurrent imaging using optical microscopy.
Statement of significance
Currently, there are limited methods for the non-invasive real-time assessment of cellular sensitivity to mechanical stimuli within 3D tissue scaffolds. We describe an original approach that utilizes a pulsed laser microbeam within a standard laser scanning microscope system to generate single cavitation bubbles to provide impulsive mechanostimulation to cells within 3D fibrillar and amorphous hydrogels. Using this technique, we measure the cellular mechanosensitivity of primary human dermal fibroblasts embedded in amorphous and fibrillar hydrogels, thereby providing a useful method to examine cellular mechanotransduction in 3D biomaterials. Moreover, the implementation of our method within a standard optical microscope makes it suitable for broad adoption by cellular mechanotransduction researchers and opens the possibility of high-throughput evaluation of biomaterials with respect to cellular mechanosignaling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


