S100A9调节M1巨噬细胞极化并加剧脂肪肝缺血再灌注损伤
IF 1.6
4区 医学
Q4 IMMUNOLOGY
引用次数: 0
摘要
目的:脂肪肝更容易受到缺血再灌注(IR)损伤,这增加了肝移植后原发性移植物无功能的风险。S100A9 被认为是调节肝病进展的关键先天性免疫传感器。然而,其在脂肪肝IR损伤中的意义仍未得到充分研究:在小鼠模型中,我们产生了 S100A9 基因敲除(S100A9 KO)小鼠,以研究 S100A9 在红外刺激脂肪肝中的作用。在体外,我们利用源自骨髓的原代巨噬细胞来探讨 S100A9 在调节巨噬细胞极化和炎症中的作用:结果:在受到红外损伤的小鼠脂肪肝中,S100A9的表达明显增加。单核细胞/巨噬细胞和中性粒细胞浸润的减少证明了 S100A9 的缺失能显著减轻肝脏炎症损伤(p<0.05)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
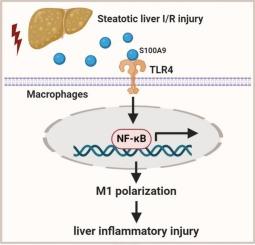
S100A9 regulates M1 macrophage polarization and exacerbates steatotic liver ischemia-reperfusion injury
Objective
Steatotic livers exhibit higher susceptibility to ischemia reperfusion (IR) injury, which increase the risk of primary graft non-function following liver transplantation. S100A9 is identified as a pivotal innate immune sensor that regulates the progression of liver diseases. However, its significance in steatotic liver IR injury remains under-investigated.
Methods
In mice model, we generated S100A9 knockout (S100A9 KO) mice to investigate the role of S100A9 in IR-stimulated steatotic livers. In vitro, primary bone marrow-derived macrophages were utilized to explore the effect of S100A9 in regulating macrophage polarization and inflammation.
Results
S100A9 expression was markedly increased in steatotic livers of mice subjected to IR insult. S100A9 deletion significantly attenuated liver inflammatory injury, as evidenced by the diminished infiltration of both monocytes/macrophages and neutrophils (p < 0.05). The expression of proinflammatory factors was reduced (p < 0.05) at the same time. Additionally, S100A9-deficient livers demonstrated M1 polarization decrease and Toll-like receptor 4 (TLR4) suppression (p < 0.05). In vitro, genetic TLR4 inhibition led to nuclear factor kappa B (NF-κB) inactivation and subsequent M1 polarization decrease (p < 0.05) in macrophages treated with recombinant S100A9. Conclusion
In this study, we highlight the pivotal role of TLR4/NF-κB as a critical mediator of S100A9 in inducing M1 macrophage polorization- dependent inflammation in steatotic livers IR injury.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Transplant immunology
医学-免疫学
CiteScore
2.10
自引率
13.30%
发文量
198
审稿时长
48 days
期刊介绍:
Transplant Immunology will publish up-to-date information on all aspects of the broad field it encompasses. The journal will be directed at (basic) scientists, tissue typers, transplant physicians and surgeons, and research and data on all immunological aspects of organ-, tissue- and (haematopoietic) stem cell transplantation are of potential interest to the readers of Transplant Immunology. Original papers, Review articles and Hypotheses will be considered for publication and submitted manuscripts will be rapidly peer-reviewed and published. They will be judged on the basis of scientific merit, originality, timeliness and quality.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


