通过转化路线合成的磁性 ZIF-8 吸附有机污染物的分子机理探析
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究介绍了一种新型磁核壳吸附剂 Fe3O4@Zeolite Imidazolate Framework-8(Fe3O4@ZIF-8)的合成过程。该工艺首先通过溶热法形成球形磁铁矿聚集体(即 Fe3O4),并使用聚丙烯酸进行稳定和封盖。然后通过溶胶凝胶过程生长出 ZIF-8 外壳,再与 2-甲基咪唑反应,形成厚度约为 60 纳米的结晶外壳。包括能量色散 X 射线分析和 X 射线衍射在内的表征技术证实了 Fe3O4@ZIF-8 的成功制备。以亚甲基蓝(MB)和双氯芬酸钠(DCF)为模型污染物,对其吸附能力进行了评估。结果表明,Fe3O4@ZIF-8 具有快速的去除率,在 15 分钟和 30 分钟内对 MB(6.01 × 10-4 g.mg-1-min-1)和 DCF(4.43 × 10-4 g-mg-1.min-1)的去除率分别达到 98%,明显优于传统活性炭。热力学研究表明,吸附过程是自发的;焓变化驱动甲基溴的吸附,而 DCF 则受熵因素的影响。分子建模揭示了 ZIF-8 不同面上的优先吸附行为,π-π 堆积和氢键对甲基溴的作用更强。这些发现强调了 Fe3O4@ZIF-8 作为一种有效的水净化吸附剂的潜力,突出了其强大的吸附能力(对 MB 的吸附能力为 73.2 mg-g-1,对 DCF 的吸附能力为 53.8 mg-g-1)、稳定性和可重复使用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

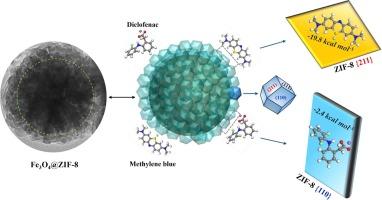
Insight into the molecular mechanism of organic pollutants’ adsorption on magnetic ZIF-8 synthesized via a transformational route
This study presents the synthesis of Fe3O4@Zeolite Imidazolate Framework-8 (Fe3O4@ZIF-8), a novel magnetic core–shell adsorbent engineered for enhance adsorption of organic pollutants. The process begins with the formation of spherical magnetite aggregates (i.e., Fe3O4) via a solvothermal method, using polyacrylic acid for stabilization and capping. A ZIF-8 shell is then grown through a sol gel process followed by reaction with 2-methylimidazole, resulting in a crystalline shell approximately 60 nm thick. Characterization techniques, including energy dispersive X-ray analysis and X-ray diffraction, confirm the successful preparation of Fe3O4@ZIF-8. The adsorption capabilities were evaluated using methylene blue (MB) and diclofenac sodium (DCF) as model pollutants. Fe3O4@ZIF-8 demonstrated rapid removal efficiencies, with 98 % removal of MB (6.01 × 10-4 mg·g−1·min−1) and DCF (4.43 × 10-4 g·mg−1.min−1) within 15 and 30 min, respectively, significantly outperforming conventional activated carbon. Thermodynamic studies indicate that the adsorption processes are spontaneous; enthalpic changes drive MB adsorption, while DCF is influenced by entropic factors. Molecular modeling reveals preferential adsorption behaviors on different ZIF-8 facets, with stronger interactions for MB due to π-π stacking and hydrogen bonding. These findings underscore the potential of Fe3O4@ZIF-8 as an effective adsorbent for water purification, highlighting its substantial adsorption capacity (73.2 mg·g−1 for MB and 53.8 mg·g−1 for DCF), stability, and reusability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


