通过钴-铜串联催化剂将二氧化碳选择性电还原为乙醇
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
选择性电催化将二氧化碳转化为多碳产品(如乙醇)是一项重大技术挑战。目前,这种反应性受到许多单位催化剂固有的 C-C 键形成缓慢的限制。在此,我们报告了一种基于地球富集元素的新型串联电催化剂,它可促进二氧化碳到乙醇的选择性转化。这种复合催化剂由锚定在导电掺氮碳上的相邻钴原子和铜原子组成。在低过电位下(E = -0.8 V,相对于可逆氢电极),该体系显示出生产乙醇的高选择性(法拉第效率 >70%),同时在 18 小时内保持其反应活性和稳定性。运算 X 射线吸收光谱显示了单位点 Cu 向作为实际活性位点的 Cu 簇的动态转变。原位红外光谱显示,在 Co 位点上形成了中间 CO,随后在 Cu 簇上发生溢出和 C-C 耦合。这种设计理念为将二氧化碳转化为多碳产品的无贵金属串联电催化剂提供了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
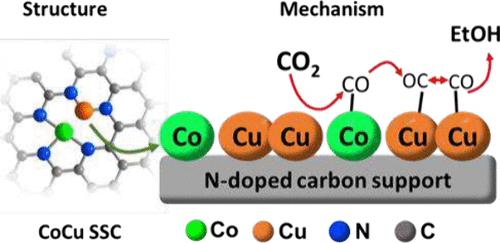
Selective Electroreduction of CO2 to Ethanol via Cobalt–Copper Tandem Catalysts
The selective electrocatalytic conversion of CO2 into multicarbon products, such as ethanol, is a major technological challenge. Currently, this reactivity is limited by the sluggish formation of C–C bonds inherent to many single-site catalysts. Here, we report a new tandem electrocatalyst based on earth-abundant elements, which facilitates the selective CO2-to-ethanol conversion. The composite catalyst consists of neighboring cobalt and copper atoms anchored to electrically conductive nitrogen-doped carbon. At low overpotentials (E = −0.8 V vs reversible hydrogen electrode), the system shows high selectivity for ethanol production (faradaic efficiencies >70%), while retaining its reactivity and stability for 18 h. A CO spillover mechanism is proposed as the basis for the observed selectivity, where efficient CO generation at Co sites leads to high local CO concentrations at neighboring Cu sites, thereby favoring C–C coupling and ethanol formation. Operando X-ray absorption spectroscopy reveals a dynamic transformation of the single-site Cu into Cu clusters as actual active sites. In situ infrared spectroscopy reveals the formation of intermediate CO at Co sites, which undergo subsequent spillover and C–C coupling on the Cu clusters. This design concept offers new avenues for noble metal-free tandem electrocatalysts for the conversion of CO2-to-multicarbon products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


