辐射诱导的胶原链破碎、交联和连续辐照对人体皮质骨高循环疲劳寿命的影响。
IF 3.3
2区 医学
Q2 ENGINEERING, BIOMEDICAL
Journal of the Mechanical Behavior of Biomedical Materials
Pub Date : 2024-09-30
DOI:10.1016/j.jmbbm.2024.106759
引用次数: 0
摘要
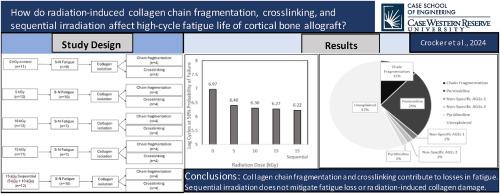
人体皮质骨异体移植的高循环疲劳寿命和抗疲劳裂纹扩展能力在 0 至 25 kGy 之间与辐射剂量有关,剂量越高,寿命越短。最近,我们发现胶原链的断裂和稳定交联的积累可能会导致人体皮质骨的抗疲劳裂纹扩展能力随辐射剂量而下降。据我们所知,这些机制对皮质骨高循环疲劳寿命的影响尚未确定。序列辐照也被证明能减轻肌腱疲劳寿命的损失,但是否能减轻骨皮质疲劳寿命的损失还没有进行过研究。我们的目标是评估在 0-15 kGy 剂量范围内辐射诱导的胶原链断裂和交联对皮质骨高循环疲劳寿命的影响,并评估 15 kGy 连续辐照是否能减轻高循环疲劳寿命损失和辐射诱导的胶原损伤。来自四对男性供体股骨的高循环疲劳寿命试样被分为 5 个处理组(0 kGy、5 kGy、10 kGy、15 kGy 和 15 kGy 连续辐照),并在 35 MPa 的循环应力下使用定制的旋转弯曲仪器进行高循环疲劳寿命测试。疲劳测试后,从疲劳试样中分离出胶原蛋白,并分别使用 SDS-PAGE 和荧光测定法对胶原蛋白链的断裂和交联累积进行量化。胶原链破碎(p = 0.006)和非酶交联(p本文章由计算机程序翻译,如有差异,请以英文原文为准。
The influence of radiation-induced collagen chain fragmentation, crosslinking, and sequential irradiation on the high-cycle fatigue life of human cortical bone
Both high-cycle fatigue life and fatigue crack propagation resistance of human cortical bone allograft are radiation dose-dependent between 0 and 25 kGy such that higher doses exhibit progressively shorter lifetimes. Recently, we have shown that collagen chain fragmentation and stable crosslink accumulation may contribute to the radiation dose-dependent loss in fatigue crack propagation resistance of human cortical bone. To our knowledge, the influence of these mechanisms on high-cycle fatigue life of cortical bone have not been established. Sequential irradiation has also been shown to mitigate the loss of fatigue life of tendons, however, whether this mitigates losses in fatigue life of cortical bone has not been explored. Our objectives were to evaluate the influence of radiation-induced collagen chain fragmentation and crosslinking on the high-cycle fatigue life of cortical bone in the dose range of 0–15 kGy, and to evaluate the capability of sequential irradiation at 15 kGy to mitigate the loss of high-cycle fatigue life and radiation-induced collagen damage. High-cycle fatigue life specimens from four male donor femoral pairs were divided into 5 treatment groups (0 kGy, 5 kGy, 10 kGy, 15 kGy, and 15 kGy sequentially irradiated) and subjected to high-cycle fatigue life testing with a custom rotating-bending apparatus at a cyclic stress of 35 MPa. Following fatigue testing, collagen was isolated from fatigue specimens, and collagen chain fragmentation and crosslink accumulation were quantified using SDS-PAGE and a fluorometric assay, respectively. Both collagen chain fragmentation (p = 0.006) and non-enzymatic crosslinking (p < 0.001) influenced high-cycle fatigue life, which decreased with increasing radiation dose from 0 to 15 kGy (p = 0.016). Sequential irradiation at 15 kGy did not offer any mitigation in high-cycle fatigue life (p = 0.93), collagen chain fragmentation (p = 0.99), or non-enzymatic crosslinking (p ≥ 0.10) compared to a single radiation dose of 15 kGy. Taken together with our previous findings on the influence of collagen damage on fatigue crack propagation resistance, collagen chain fragmentation and crosslink accumulation both contribute to radiation-induced losses in notched and unnotched fatigue life of cortical bone. To maximize the functional lifetime of radiation sterilized structural cortical bone allografts, pathways other than sequential radiation should be explored to mitigate collagen matrix damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of the Mechanical Behavior of Biomedical Materials
工程技术-材料科学:生物材料
CiteScore
7.20
自引率
7.70%
发文量
505
审稿时长
46 days
期刊介绍:
The Journal of the Mechanical Behavior of Biomedical Materials is concerned with the mechanical deformation, damage and failure under applied forces, of biological material (at the tissue, cellular and molecular levels) and of biomaterials, i.e. those materials which are designed to mimic or replace biological materials.
The primary focus of the journal is the synthesis of materials science, biology, and medical and dental science. Reports of fundamental scientific investigations are welcome, as are articles concerned with the practical application of materials in medical devices. Both experimental and theoretical work is of interest; theoretical papers will normally include comparison of predictions with experimental data, though we recognize that this may not always be appropriate. The journal also publishes technical notes concerned with emerging experimental or theoretical techniques, letters to the editor and, by invitation, review articles and papers describing existing techniques for the benefit of an interdisciplinary readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


