探索生物医学应用中三重周期性最小表面结构的最佳机械性能:数值分析
IF 3.3
2区 医学
Q2 ENGINEERING, BIOMEDICAL
Journal of the Mechanical Behavior of Biomedical Materials
Pub Date : 2024-09-30
DOI:10.1016/j.jmbbm.2024.106757
引用次数: 0
摘要
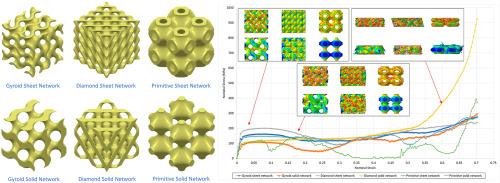
目前,选择性激光熔融(SLM)和电子束熔融(EBM)等尖端增材制造技术为制造商提供了一条宝贵的途径,尤其是在生物医学设备方面。这些技术能制造出复杂的多孔结构,这些结构从大自然中汲取灵感,具有生物兼容性,能有效解决与固体植入物相关的不利问题,包括应力屏蔽、皮质肥厚和微动。在此类多孔结构领域,三周期极小表面(TPMS)结构,特别是陀螺型、钻石型和原始型设计,因其生物启发形式和卓越的机械和疲劳特性而表现出非凡的性能,超越了其他多孔结构。因此,它们成为生物医学植入物的有力竞争者。然而,评估 TPMS 结构在适合生物医学应用的孔径、单胞尺寸和孔隙率范围内的机械性能和可制造性仍然至关重要。本研究的目的是在适合细胞播种、血管化和骨结合等任务的形态参数范围内,仔细研究Gyroid、Diamond和Primitive结构在实体和片状网络迭代中的机械行为。此外,还与宿主骨骼的机械特性进行了比较。该方法围绕有限元法(FEM)进行分析。这六种结构的最初建模单元尺寸分别为 1、1.5、2 和 2.5 毫米,孔隙率从 50%到 85%不等。随后,通过数值分析量化了弹性模量和屈服强度等力学性能。研究结果表明,采用 TPMS 设计可实现 1 至 2.5 毫米之间的单胞尺寸,使孔隙尺寸在生物医学植入物的合适范围(约 300-1500 μm)内。在 50%-85%的孔隙率范围内,弹性模量介于 1.5 至 33.8 GPa 之间,屈服强度约为 20-304.5 MPa。一般来说,在上述范围内,改变单胞尺寸对机械性能的影响极小;但值得注意的是,孔隙率越小,加成制造结构中的缺陷就越多。因此,对于可接受的 500-1000 μm 的孔径范围和 150 μm 的最小壁厚,谨慎的选择是采用 2.5 mm 的单元格尺寸。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the optimal mechanical properties of triply periodic minimal surface structures for biomedical applications: A Numerical analysis
Currently, cutting-edge Additive Manufacturing techniques, such as Selective Laser Melting (SLM) and Electron Beam Melting (EBM), offer manufacturers a valuable avenue, especially in biomedical devices. These techniques produce intricate porous structures that draw inspiration from nature, boast biocompatibility, and effectively counter the adverse issues tied to solid implants, including stress shielding, cortical hypertrophy, and micromotions. Within the domain of such porous structures, Triply Periodic Minimal Surface (TPMS) configurations, specifically the Gyroid, Diamond, and Primitive designs, exhibit exceptional performance due to their bioinspired forms and remarkable mechanical and fatigue properties, outshining other porous counterparts. Consequently, they emerge as strong contenders for biomedical implants. However, assessing the mechanical properties and manufacturability of TPMS structures within the appropriate ranges of pore size, unit cell size, and porosity tailored for biomedical applications remains paramount. This study aims to scrutinize the mechanical behavior of Gyroid, Diamond, and Primitive structures in solid and sheet network iterations within the morphological parameter ranges suitable for tasks like cell seeding, vascularization, and osseointegration. A comparison with the mechanical characteristics of host bones is also undertaken. The methodology revolves around Finite Element Method (FEM) analysis. The six structures are originally modeled with unit cell sizes of 1, 1.5, 2, and 2.5 mm, and porosity levels ranging from 50% to 85%. Subsequently, mechanical properties, such as elasticity modulus and yield strength, are quantified through numerical analysis. The results underscore that implementing TPMS designs enables unit cell sizes between 1 and 2.5 mm, facilitating pore sizes within the suitable range of approximately 300–1500 μm for biomedical implants. Elasticity modulus spans from 1.5 to 33.8 GPa, while yield strength ranges around 20–304.5 MPa across the 50%–85% porosity spectrum. Generally, altering the unit cell size exhibits minimal impact on mechanical properties within the range above; however, it's noteworthy that smaller porosities correspond to heightened defects in additively manufactured structures. Thus, for an acceptable pore size range of 500–1000 μm and a minimum wall thickness of 150 μm, a prudent choice would involve adopting a 2.5 mm unit cell size.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of the Mechanical Behavior of Biomedical Materials
工程技术-材料科学:生物材料
CiteScore
7.20
自引率
7.70%
发文量
505
审稿时长
46 days
期刊介绍:
The Journal of the Mechanical Behavior of Biomedical Materials is concerned with the mechanical deformation, damage and failure under applied forces, of biological material (at the tissue, cellular and molecular levels) and of biomaterials, i.e. those materials which are designed to mimic or replace biological materials.
The primary focus of the journal is the synthesis of materials science, biology, and medical and dental science. Reports of fundamental scientific investigations are welcome, as are articles concerned with the practical application of materials in medical devices. Both experimental and theoretical work is of interest; theoretical papers will normally include comparison of predictions with experimental data, though we recognize that this may not always be appropriate. The journal also publishes technical notes concerned with emerging experimental or theoretical techniques, letters to the editor and, by invitation, review articles and papers describing existing techniques for the benefit of an interdisciplinary readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


