与同域的本地水禽相比,入侵埃及雁的适应性免疫力较低。
IF 2.1
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology
Pub Date : 2024-10-02
DOI:10.1016/j.cbpa.2024.111752
引用次数: 0
摘要
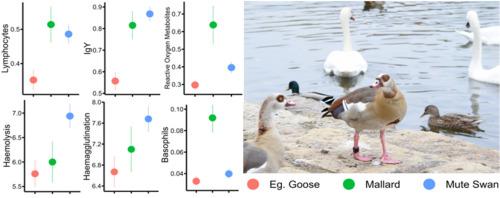
成功的入侵物种会将用于繁殖和扩大范围的营养和代谢资源与其他代价高昂的身体功能进行权衡,从而提高传播的成功率。重新分配资源的一个拟议机制是权衡免疫功能和氧化状态的调节。通过对德国的一个入侵物种(埃及鹅)和两个本地竞争物种(野鸭和疣鼻天鹅)的免疫功能和氧化状态的血液标记进行量化,我们检验了以下假设:与本地物种相比,入侵物种(i)在免疫功能方面的投资较低;(ii)氧化损伤水平较低;(iii)没有较高的抗氧化防御能力。我们发现,与两个本地物种相比,入侵物种的适应性免疫标志物(淋巴细胞和免疫球蛋白 Y)水平较低。埃及雁和野鸭的先天免疫特征基本相似。相反,与埃及雁和野鸭相比,疣鼻天鹅的嗜异性细胞和溶菌酶水平较高,细菌杀伤能力较低。与埃及雁相比,疣鼻天鹅的溶血和血凝水平较高,但单核细胞和血细胞比容水平较低。与其他水禽物种相比,野鸭的氧化损伤标志物--活性氧代谢物含量较高,而埃及雁的含量较低,而这三种水禽的抗氧化剂含量基本相似。我们的研究结果表明,入侵物种对适应性免疫功能的投资减少,这可能是一种节约资源的免疫策略,因为在新的定殖栖息地失去了共同进化的寄生虫。降低对免疫功能的投资可能有利于其他需要能量的活动,如繁殖、扩散和领地性,而保持相对较高的先天免疫力则是有益的,因为入侵物种主要会遇到新的病原体。研究结果还指出了基线免疫状态中其他重要的物种特异性差异,支持了之前关于物种体重与免疫状况之间关系的研究结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lower adaptive immunity in invasive Egyptian geese compared to sympatric native waterfowls
Successful invasive species increase their spreading success by trading-off nutritional and metabolic resources allocated to reproduction and range expansion with other costly body functions. One proposed mechanism for the reallocation of resources is a trade-off with the immune function and the regulation of oxidative status. Relying on a panel of blood-based markers of immune function and oxidative status quantified in an invasive species (Egyptian goose) and two native competing species (mallard and mute swan) in Germany, we tested the hypothesis that the invasive species would have (i) lower investment in immune function, (ii) lower levels of oxidative damage, and (iii) no higher antioxidant defences compared to the native species. We found lower levels of adaptive immune markers (lymphocytes and immunoglobulin Y), in the invasive species compared to the two native species. Innate immune profile was generally similar between Egyptian geese and mallards. By contrast, mute swans showed higher levels of heterophils and lysozymes, and lower levels of bacteria killing ability compared to both Egyptian geese and mallards. Mute swans also showed higher levels of haemolysis and haemagglutination, but lower levels of monocytes and haematocrit compared to Egyptian geese. Reactive oxygen metabolites, a marker of oxidative damage, were higher in mallards and lower in Egyptian geese compared to the other waterfowl species, while levels of antioxidants were generally similar among the three species. Our results point to a reduced investment in adaptive immune function in the invasive species as a possible resources-saving immunological strategy due to the loss of co-evolved parasites in the new colonised habitats, as observed in a previous study. A lower investment in immune function may benefit other energy-demanding activities, such as reproduction, dispersal, and territoriality, while maintaining relatively higher innate immunity is beneficial since invasive species mainly encounter novel pathogens. Results pointed out also other important species-specific differences in baseline immune status, supporting previous findings on the relationship between species' body mass and immune profile.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.00
自引率
4.30%
发文量
155
审稿时长
3 months
期刊介绍:
Part A: Molecular & Integrative Physiology of Comparative Biochemistry and Physiology. This journal covers molecular, cellular, integrative, and ecological physiology. Topics include bioenergetics, circulation, development, excretion, ion regulation, endocrinology, neurobiology, nutrition, respiration, and thermal biology. Study on regulatory mechanisms at any level of organization such as signal transduction and cellular interaction and control of behavior are also published.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


