将水蛭素封装在 pH 值响应型抗氧化剂纳米颗粒中,对缺血性中风模型小鼠产生疗效。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
本研究介绍了一种新型 pH 值敏感的水蛭素负载抗氧化纳米粒子(HD@iNanoAOX),旨在解决水蛭素半衰期短和出血转化的难题。HD@iNanoAOX 的设计原理是将水蛭素包裹在抗氧化纳米颗粒中,以保护和延长其生物活性,促进其在酸性环境中的逐步释放。体内外实验验证了这种方法的功效。体内外溶栓试验表明,HD@iNanoAOX 在酸性条件下仍能保持有效的血栓溶解活性。体内评估显示,在大脑中动脉闭塞(MCAO)小鼠模型中,HD@iNanoAOX 能显著延长水蛭素的半衰期并缩小脑梗塞体积。此外,HD@iNanoAOX 治疗还能减轻脑氧化应激,抑制出血转化,防止血脑屏障(BBB)破坏。这些研究结果表明,HD@iNanoAOX 的溶栓和抗氧化特性为缺血性中风提供了一种前景广阔的治疗方法。不过,还需要进一步研究,以优化配方并评估其在临床环境中的安全性和有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
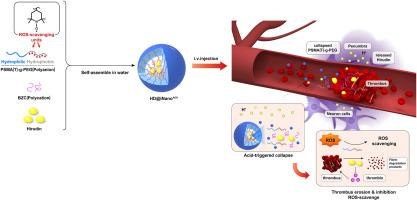
Engineering hirudin encapsulation in pH-responsive antioxidant nanoparticles for therapeutic efficacy in ischemic stroke model mice
This study introduces a novel pH-sensitive, hirudin-loaded antioxidant nanoparticle (HD@iNanoAOX) aimed at addressing the challenges of hirudin's short half-life and hemorrhagic transformation. HD@iNanoAOX was engineered to safeguard and prolong hirudin's bioactivity by encapsulating it within antioxidative nanoparticles, facilitating its gradual release in acidic environments. The efficacy of this approach was validated through both ex vivo and in vivo experiments. Ex vivo thrombolytic assays demonstrated that HD@iNanoAOX maintained effective clot lysis activity under acidic conditions. In vivo assessments revealed that HD@iNanoAOX significantly prolonged hirudin's half-life and reduced cerebral infarct volume in a mouse model of middle cerebral artery occlusion (MCAO). Furthermore, HD@iNanoAOX treatment mitigated cerebral oxidative stress, suppressed hemorrhagic transformation, and prevented blood-brain barrier (BBB) disruption. These findings suggest that the combined thrombolytic and antioxidative properties of HD@iNanoAOX offer a promising therapeutic approach for ischemic stroke. Nonetheless, further research is warranted to optimize the formulation and assess its safety and efficacy in clinical settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


