通过碳-碳键裂解从木质素中获取单体
IF 51.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
木质素是维管植物细胞壁中的异质芳香族大分子,是生产生物化学品和生物燃料的丰富原料。由于木质素中含有大量的 β-O-4 键,而且有大量可用的 C-O 键裂解策略,因此几十年来的研究重点都是通过 C-O 键裂解来获得单体。然而,由于存在难处理的 C-C 键,其选择性裂解仍是催化过程中的一大挑战,因此单体产量总是低于预期。在本综述中,我们将重点介绍木质素 C-C 裂解反应,包括生物合成(β-1、β-5、β-β 和 5-5)和工业加工(5-CH2-5 和 α-5)过程中产生的连接。我们研究了 C-C 裂解的多种方法,包括均相催化和异相催化、光催化和生物催化,以确定有前途的进一步研究策略,并为木质素 C-C 键裂解的明确测量提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
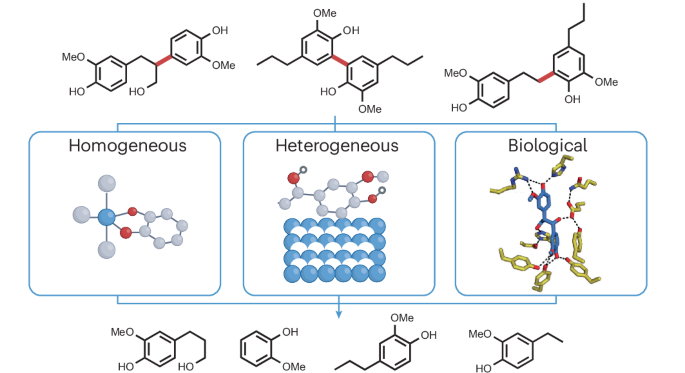
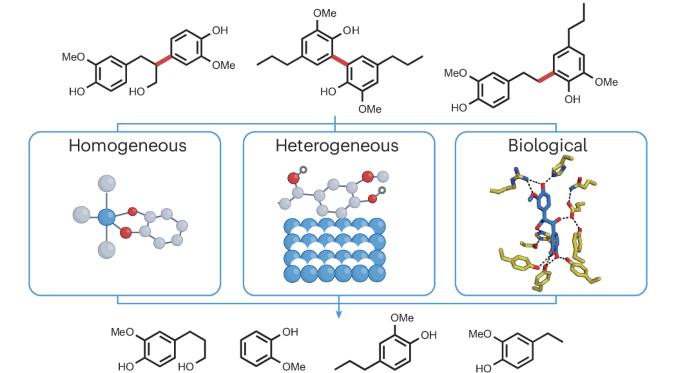
Accessing monomers from lignin through carbon–carbon bond cleavage
Lignin, the heterogeneous aromatic macromolecule found in the cell walls of vascular plants, is an abundant feedstock for the production of biochemicals and biofuels. Many valorization schemes rely on lignin depolymerization, with decades of research focused on accessing monomers through C–O bond cleavage, given the abundance of β–O–4 bonds in lignin and the large number of available C–O bond cleavage strategies. Monomer yields are, however, invariably lower than desired, owing to the presence of recalcitrant C–C bonds whose selective cleavage remains a major challenge in catalysis. In this Review, we highlight lignin C–C cleavage reactions, including those of linkages arising from biosynthesis (β–1, β–5, β–β and 5–5) and industrial processing (5–CH2–5 and α–5). We examine multiple approaches to C–C cleavage, including homogeneous and heterogeneous catalysis, photocatalysis and biocatalysis, to identify promising strategies for further research and provide guidelines for definitive measurements of lignin C–C bond cleavage. To date, monomer yields from lignin are limited to those attainable through C–O bond cleavage. Cleaving C–C bonds often leads to deleterious product degradation and low monomer yields. Herein we review lignin C–C cleavage reports and advocate for a standardized reporting of yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


