Rubisco 为 2-C-methyl-d-erythritol-4-phosphate 途径提供丙酮酸
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
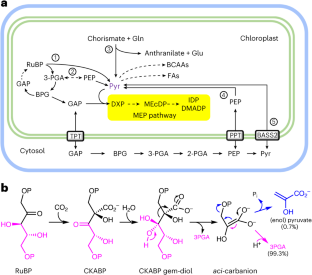
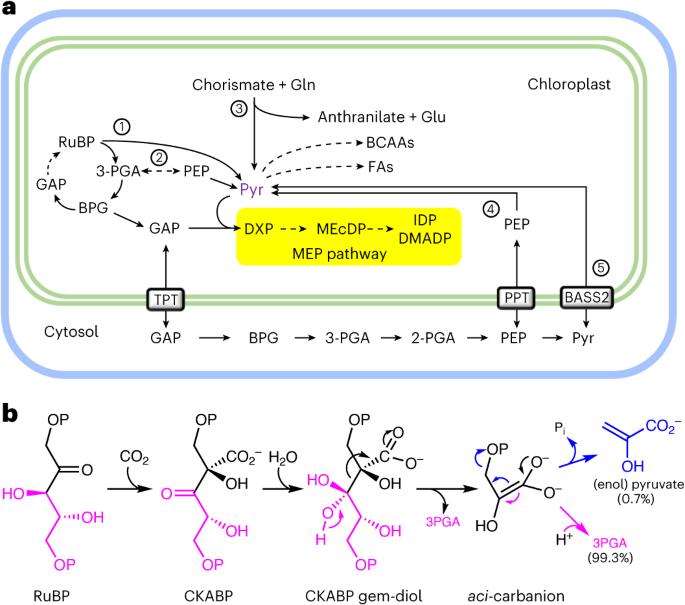
RIBULOSE-1,5-BISPHOSPHATE-CARBOXYLASE/OXYGENASE(Rubisco)通过β-消除aci-carbanion 中间体1 在叶绿体中产生丙酮酸。在这里,我们展示了这一副反应为光合作用活跃组织中异戊二烯、脂肪酸和支链氨基酸的生物合成提供丙酮酸。对完整拟南芥植株进行的 13C 标记研究表明,丙酮酸的总碳承载量太大,磷酸烯醇丙酮酸无法作为前体。低氧会刺激 Rubisco 羧化酶的活性,增加丙酮酸的产生和通过 2-C-甲基-d-赤藓糖醇-4-磷酸(MEP)途径的通量,从而为质体异戊二烯的生物合成提供前体2,3。对磷酸烯醇丙酮酸或丙酮酸输入缺陷突变体的代谢组分析以及对分离叶绿体的生化鉴定进一步证明 Rubisco 是叶绿体中丙酮酸的主要来源。幼苗将外源 13C 标记的丙酮酸纳入 MEP 途径中间产物,而成年植株则没有,这突出表明了丙酮酸来源的发育转变。在体外试验中,Rubisco β-消除导致的丙酮酸占产物谱的 0.7%,这相当于离开卡尔文-本森-巴塞尔循环的总碳量的 2%。这些发现解决了 "丙酮酸悖论 "4 ,改进了中心代谢模型的拟合,并将 MEP 途径与碳同化直接联系起来。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rubisco supplies pyruvate for the 2-C-methyl-d-erythritol-4-phosphate pathway
RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE/OXYGENASE (Rubisco) produces pyruvate in the chloroplast through β-elimination of the aci-carbanion intermediate1. Here we show that this side reaction supplies pyruvate for isoprenoid, fatty acid and branched-chain amino acid biosynthesis in photosynthetically active tissue. 13C labelling studies of intact Arabidopsis plants demonstrate that the total carbon commitment to pyruvate is too large for phosphoenolpyruvate to serve as a precursor. Low oxygen stimulates Rubisco carboxylase activity and increases pyruvate production and flux through the 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway, which supplies the precursors for plastidic isoprenoid biosynthesis2,3. Metabolome analysis of mutants defective in phosphoenolpyruvate or pyruvate import and biochemical characterization of isolated chloroplasts further support Rubisco as the main source of pyruvate in chloroplasts. Seedlings incorporated exogenous,13C-labelled pyruvate into MEP pathway intermediates, while adult plants did not, underscoring the developmental transition in pyruvate sourcing. Rubisco β-elimination leading to pyruvate constituted 0.7% of the product profile in in vitro assays, which translates to 2% of the total carbon leaving the Calvin–Benson–Bassham cycle. These insights solve the “pyruvate paradox”4, improve the fit of metabolic models for central metabolism and connect the MEP pathway directly to carbon assimilation. As nature’s most important enzyme, Rubisco fixes carbon dioxide in the Calvin–Benson–Bassham cycle. Its lesser-known side job is supplying pyruvate in the chloroplast, an observation that solves a long-standing paradox of central metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


