核定位丙酮酸激酶控制组蛋白 H3 苏氨酸 11 上的磷酸化
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
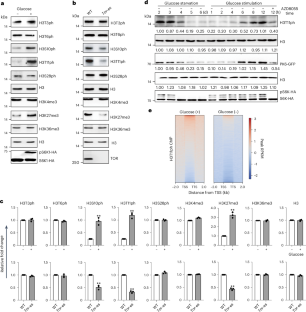
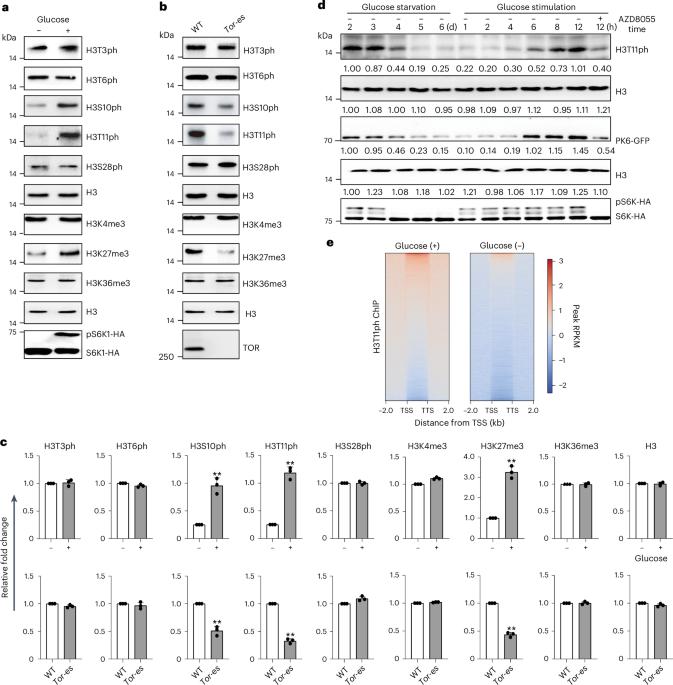
组蛋白 H3 在苏氨酸 11 处的磷酸化(H3T11ph)会影响转录和染色体的稳定性。然而,植物中负责沉积 H3T11ph 的酶和 H3T11ph 的功能仍然未知。在这里,我们报告了拟南芥中的吡咯烷酮激酶 6(PK6)、PK7 和 PK8 在充足的葡萄糖和光照条件下进入细胞核,它们在细胞核中与 SWI2/SNF2-RELATED 1 COMPLEX 4(SWC4)相互作用并在苏氨酸 11 处磷酸化 H3。这些激酶的突变或 SWC4 的敲除会导致 FLC 依赖性早花、下胚轴短和花梗短。在全基因组范围内,H3T11ph高度富集于转录起始位点和转录终止位点,并与基因转录水平呈正相关。PK6 和 SWC4 以 FLC、MYB73、PRE1、TCP4 和 TCP10 为靶标,在这些位点沉积 H3T11ph 并促进其转录,PK6 在这些位点的占据需要 SWC4。我们的研究结果表明,核定位的 PK6、PK7 和 PK8 可调节 H3T11ph 和植物生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nuclear-localized pyruvate kinases control phosphorylation of histone H3 on threonine 11
Phosphorylation of histone H3 at threonine 11 (H3T11ph) affects transcription and chromosome stability. However, the enzymes responsible for depositing H3T11ph and the functions of H3T11ph in plants remain unknown. Here we report that in Arabidopsis thaliana, PYRUVATE KINASE 6 (PK6), PK7 and PK8 enter the nucleus under conditions of sufficient glucose and light exposure, where they interact with SWI2/SNF2-RELATED 1 COMPLEX 4 (SWC4) and phosphorylate H3 at threonine 11. Mutations in these kinases or knockdown of SWC4 resulted in FLC-dependent early flowering, short hypocotyls and short pedicels. Genome-wide, H3T11ph is highly enriched at transcription start sites and transcription termination sites, and positively correlated with gene transcript levels. PK6 and SWC4 targeted FLC, MYB73, PRE1, TCP4 and TCP10, depositing H3T11ph at these loci and promoting their transcription, and PK6 occupancy at these loci requires SWC4. Together, our results reveal that nuclear-localized PK6, PK7 and PK8 modulate H3T11ph and plant growth. This study reports that the nucleocytoplasmic shuttling of pyruvate kinase 6 (PK6), PK7 and PK8 mediates phosphorylation of H3 at threonine 11, represses flowering time, and promotes hypocotyl and pedicel elongation in Arabidopsis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


