植物 PR1 挽救了真菌效应物对质体铁硫蛋白的凝结作用
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
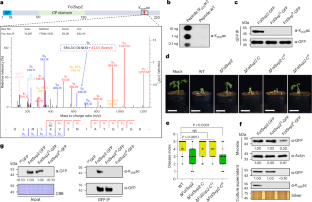
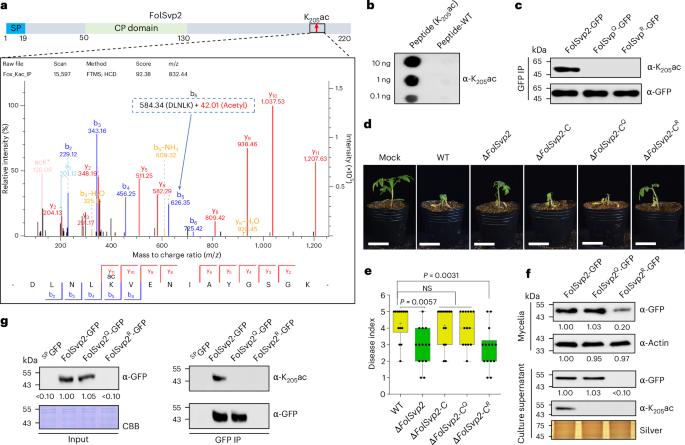
植物病原体会分泌大量效应物来促进宿主感染,但这些毒性蛋白是否会发生相分离以操纵植物防御,以及宿主如何应对这一事件仍是未知数。在这里,我们发现真菌病原体 Fusarium oxysporum f. sp. lycopersici(Fol)分泌的效应子 FolSvp2 通过相分离将番茄铁硫蛋白(SlISP)从质体转运到植物体内的效应子凝聚体中。SlISP 的转移会减少植物活性氧的产生,从而促进 Fol 的入侵。FolSvp2 的作用还需要 K205 乙酰化,以防止该蛋白在 Fol 和植物细胞中发生泛素依赖性降解。然而,番茄进化出了一种防御蛋白--SlPR1。细胞外 SlPR1 与 FolSvp2 发生物理作用,抑制其进入宿主细胞,从而消除其有害作用。这些发现揭示了 PR1 在对抗一种新的效应器作用模式方面的未知功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Plant PR1 rescues condensation of the plastid iron-sulfur protein by a fungal effector
Plant pathogens secrete numerous effectors to promote host infection, but whether any of these toxic proteins undergoes phase separation to manipulate plant defence and how the host copes with this event remain elusive. Here we show that the effector FolSvp2, which is secreted from the fungal pathogen Fusarium oxysporum f. sp. lycopersici (Fol), translocates a tomato iron-sulfur protein (SlISP) from plastids into effector condensates in planta via phase separation. Relocation of SlISP attenuates plant reactive oxygen species production and thus facilitates Fol invasion. The action of FolSvp2 also requires K205 acetylation that prevents ubiquitination-dependent degradation of this protein in both Fol and plant cells. However, tomato has evolved a defence protein, SlPR1. Apoplastic SlPR1 physically interacts with and inhibits FolSvp2 entry into host cells and, consequently, abolishes its deleterious effect. These findings reveal a previously unknown function of PR1 in countering a new mode of effector action. There is a continuous arms race between pathogens and their host plants. Li et al. reveal that PR1 prevents entry of a fungal effector into plant cells from the apoplast that otherwise would quench host defence oxidizing agents via phase separation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


