利用微吸管抽吸技术评估软细胞聚集体流变性的双组分模型。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
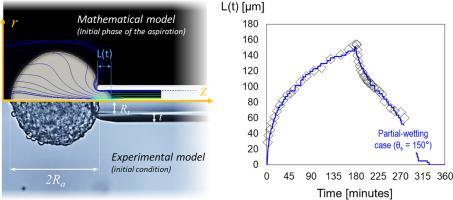
微量移液管吸液技术是用于表征惰性流体和生物软物质(如细胞聚集体)物理性质的经典实验。流体的物理参数,如粘度和界面张力,是通过研究当吸入压力增加时流体如何进入移液管,以及当吸入压力为零时流体如何松弛而获得的。要推断类流体物质的物理参数,需要一个能代表实验的数学模型;然而,对于细胞或细胞聚集体等生物材料,这些模型总是基于强烈的起始假设,而这些假设会影响已确定参数的意义。在本文中,我们从细胞聚集体的双成分性质出发,推导出一个基于卡恩-希利亚德-纳维尔-斯托克斯方程组的通用数学模型。该模型用于定量描述细胞聚集体吸入和吸出移液管的动态过程。我们通过对两种情况的研究,证明了模型的预测能力,并强调了假设对确定参数的影响:一种是细胞与移液管壁之间的非润湿条件(文献中的经典假设),另一种是更现实的部分润湿条件(接触角 θs = 150°)。此外,我们的研究结果为吸入和回缩反应之间的不对称性提供了纯物理的解释,这与所提出的细胞聚集体表面张力的机械反应改变假说不同。意义说明:我们的研究引入了一个基于卡恩-希利亚德-纳维尔-斯托克斯方程的通用数学模型,专门用于模拟细胞聚集体的微吸管吸液。该模型考虑了细胞聚集体的多组分结构及其固有的粘弹性流变学。通过挑战现有假设,特别是有关完美非润湿条件和细胞表面张力的机械响应变化的假设,我们证明了数学模型的可靠性,并阐明了其中的作用机制,为实验中观察到的吸入和回缩阶段不对称现象提供了纯粹的物理解释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A bi-component model to assess the rheology of soft cellular aggregates probed using the micropipette aspiration technique
The micro-pipette aspiration technique is a classical experiment used to characterize the physical properties of inert fluids and biological soft materials such as cellular aggregates. The physical parameters of the fluid, as viscosity and interfacial tension, are obtained by studying how the fluid enters the pipette when the suction pressure is increased and how it relaxes when the suction pressure is put to zero. A mathematical model representative of the experiment is needed to extrapolate the physical parameters of the fluid-like matter; however, for biological materials as cells or cell aggregates mathematical models are always based on strong starting hypotheses that impact the significance of the identified parameters. In this article, starting from the bi-constituent nature of the cell aggregate, we derive a general mathematical model based of a Cahn–Hilliard–Navier–Stokes set of equations. The model is applied to describe quantitatively the aspiration-retraction dynamics of a cell-aggregate into and out of a pipette. We demonstrate the predictive capability of the model and highlight the impact of the assumptions made on the identified parameters by studying two cases: one with a non-wetting condition between the cells and the wall of the pipette (classical assumption in the literature) and the second one, which is more realistic, with a partial wetting condition (contact angle θs = 150°). Furthermore, our results provide a purely physical explanation to the asymmetry between the aspiration and retraction responses which is alternative to the proposed hypothesis of an mechano-responsive alteration of the surface tension of the cell aggregate.
Statement of significance
Our study introduces a general mathematical model, based on the Cahn-Hilliard-Navier-Stokes equations, tailored to model micro-pipette aspiration of cell aggregates. The model accounts for the multi-component structure of the cell aggregate and its intrinsic viscoelastic rheology. By challenging prevailing assumptions, particularly regarding perfect non-wetting conditions and the mechano-responsive alteration of cell surface tension, we demonstrate the reliability of the mathematical model and elucidate the mechanisms at play, offering a purely physical explanation for observed asymmetries between the aspiration and retraction stages of the experiment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


